Quorum sensing in group A Streptococcus
- PMID: 25309879
- PMCID: PMC4162386
- DOI: 10.3389/fcimb.2014.00127
Quorum sensing in group A Streptococcus
Abstract
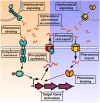
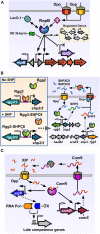
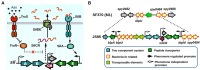
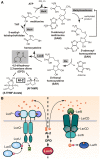
Quorum sensing (QS) is a widespread phenomenon in the microbial world that has important implications in the coordination of population-wide responses in several bacterial pathogens. In Group A Streptococcus (GAS), many questions surrounding QS systems remain to be solved pertaining to their function and their contribution to the GAS lifestyle in the host. The QS systems of GAS described to date can be categorized into four groups: regulator gene of glucosyltransferase (Rgg), Sil, lantibiotic systems, and LuxS/AI-2. The Rgg family of proteins, a conserved group of transcription factors that modify their activity in response to signaling peptides, has been shown to regulate genes involved in virulence, biofilm formation and competence. The sil locus, whose expression is regulated by the activity of signaling peptides and a putative two-component system (TCS), has been implicated on regulating genes involved with invasive disease in GAS isolates. Lantibiotic regulatory systems are involved in the production of bacteriocins and their autoregulation, and some of these genes have been shown to target both bacterial organisms as well as processes of survival inside the infected host. Finally AI-2 (dihydroxy pentanedione, DPD), synthesized by the LuxS enzyme in several bacteria including GAS, has been proposed to be a universal bacterial communication molecule. In this review we discuss the mechanisms of these four systems, the putative functions of their targets, and pose critical questions for future studies.
Keywords: AI-2; Rgg; Sil; Streptococcus pyogenes; cell-cell signaling; lantibiotics; pheromones; quorum sensing.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

