Emerging electrochemical energy conversion and storage technologies
- PMID: 25309898
- PMCID: PMC4174133
- DOI: 10.3389/fchem.2014.00079
Emerging electrochemical energy conversion and storage technologies
Abstract
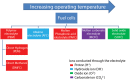
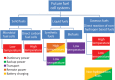
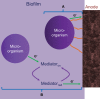
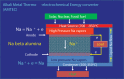
Electrochemical cells and systems play a key role in a wide range of industry sectors. These devices are critical enabling technologies for renewable energy; energy management, conservation, and storage; pollution control/monitoring; and greenhouse gas reduction. A large number of electrochemical energy technologies have been developed in the past. These systems continue to be optimized in terms of cost, life time, and performance, leading to their continued expansion into existing and emerging market sectors. The more established technologies such as deep-cycle batteries and sensors are being joined by emerging technologies such as fuel cells, large format lithium-ion batteries, electrochemical reactors; ion transport membranes and supercapacitors. This growing demand (multi billion dollars) for electrochemical energy systems along with the increasing maturity of a number of technologies is having a significant effect on the global research and development effort which is increasing in both in size and depth. A number of new technologies, which will have substantial impact on the environment and the way we produce and utilize energy, are under development. This paper presents an overview of several emerging electrochemical energy technologies along with a discussion some of the key technical challenges.
Keywords: batteries; electrochemical energy systems; electrochemical reactors; energy; energy conversion; energy storage; fuel cells.
Figures
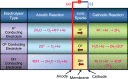
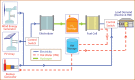
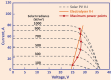

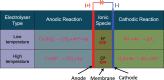
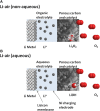
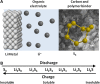

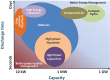

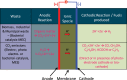

References
-
- Abraham K. M., Jiang Z. (1996). Preparation and electrochemical characterization of micron-sized spinel LiMn2O4. J. Electrochem. Soc. 143, 1–5 10.1149/1.1836378 - DOI
-
- Aeshala L. M. (2013). Effect of cationic and anionic solid polymer electrolyte on direct electrochemical reduction of gaseous CO2 to fuel. J. CO2 Util. 3–4, 49–55 10.1016/j.jcou.2013.09.004 - DOI
-
- Akhil A. A., Huff G., Currier A. B., Kaun B. C., Rastler D. M., Chen S. B., et al. (2013). DOE/EPRI 2013 Electricity Storage Handbook in Collaboration with NRECA. Albuquerque, NM: Sandia National Laboratories, SAND2013–5131
-
- Alexander B. R., Mitchell R. E., Gür T. M. (2011). Steam-carbon fuel cell concept for cogeneration of hydrogen and electrical power. J. Electrochem. Soc. 158, B505–B513 10.1149/1.3560475 - DOI
-
- Amar I. A., Lan R., Petit C. T. G., Tao S. (2011). Solid-state electrochemical synthesis of ammonia: a review. J. Solid State Electrochem. 15, 1845–1860 10.1007/s10008-011-1376-x - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

