Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia
- PMID: 25310001
- PMCID: PMC4195593
- DOI: 10.1371/journal.pone.0105311
Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia
Abstract
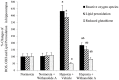
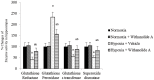
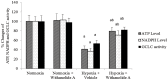
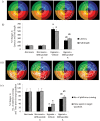
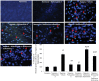
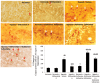
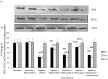
Withania somnifera root extract has been used traditionally in ayurvedic system of medicine as a memory enhancer. Present study explores the ameliorative effect of withanolide A, a major component of withania root extract and its molecular mechanism against hypoxia induced memory impairment. Withanolide A was administered to male Sprague Dawley rats before a period of 21 days pre-exposure and during 07 days of exposure to a simulated altitude of 25,000 ft. Glutathione level and glutathione dependent free radicals scavenging enzyme system, ATP, NADPH level, γ-glutamylcysteinyl ligase (GCLC) activity and oxidative stress markers were assessed in the hippocampus. Expression of apoptotic marker caspase 3 in hippocampus was investigated by immunohistochemistry. Transcriptional alteration and expression of GCLC and Nuclear factor (erythroid-derived 2)-related factor 2 (Nrf2) were investigated by real time PCR and immunoblotting respectively. Exposure to hypobaric hypoxia decreased reduced glutathione (GSH) level and impaired reduced gluatathione dependent free radical scavenging system in hippocampus resulting in elevated oxidative stress. Supplementation of withanolide A during hypoxic exposure increased GSH level, augmented GSH dependent free radicals scavenging system and decreased the number of caspase and hoescht positive cells in hippocampus. While withanolide A reversed hypoxia mediated neurodegeneration, administration of buthionine sulfoximine along with withanolide A blunted its neuroprotective effects. Exogenous administration of corticosterone suppressed Nrf2 and GCLC expression whereas inhibition of corticosterone synthesis upregulated Nrf2 as well as GCLC. Thus present study infers that withanolide A reduces neurodegeneration by restoring hypoxia induced glutathione depletion in hippocampus. Further, Withanolide A increases glutathione biosynthesis in neuronal cells by upregulating GCLC level through Nrf2 pathway in a corticosterone dependenet manner.
Conflict of interest statement
Figures


References
-
- Bahrke M, Hale BS (1993) Effect of altitude on mood, behavior and cognitive functioning. Sports Med 16: 97–125. - PubMed
-
- Baitharu I, Jain V, Deep SN, Sahu JK, Naik PK, et al. (2013) Exposure to hypobaric hypoxia and reoxygenation induces transient anxiety like behaviour in rat. J Behav Brain Sci 3: 591–602.
-
- Won SJ, Kim DY, Gwag BJ (2002) Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol 35: 67–86. - PubMed
-
- Barker JE, Heales SJR, Cassidy A, Bolanos JP, Land JM, et al. (1996) Depletion of brain glutathione results in a decrease in glutathione reductase activity, an enzyme susceptible to oxidative damage. Brain Res 716: 118–122. - PubMed
-
- Maiti P, Singh SB, Sharma AK, Muthuraju S, Banerjee PK, et al. (2006) Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem International 49: 709–716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

