Exopolysaccharide biosynthesis enables mature biofilm formation on abiotic surfaces by Herbaspirillum seropedicae
- PMID: 25310013
- PMCID: PMC4195743
- DOI: 10.1371/journal.pone.0110392
Exopolysaccharide biosynthesis enables mature biofilm formation on abiotic surfaces by Herbaspirillum seropedicae
Abstract
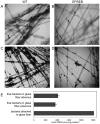
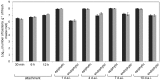
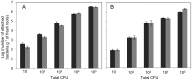
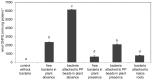
H. seropedicae associates endophytically and epiphytically with important poaceous crops and is capable of promoting their growth. The molecular mechanisms involved in plant colonization by this microrganism are not fully understood. Exopolysaccharides (EPS) are usually necessary for bacterial attachment to solid surfaces, to other bacteria, and to form biofilms. The role of H. seropedicae SmR1 exopolysaccharide in biofilm formation on both inert and plant substrates was assessed by characterization of a mutant in the espB gene which codes for a glucosyltransferase. The mutant strain was severely affected in EPS production and biofilm formation on glass wool. In contrast, the plant colonization capacity of the mutant strain was not altered when compared to the parental strain. The requirement of EPS for biofilm formation on inert surface was reinforced by the induction of eps genes in biofilms grown on glass and polypropylene. On the other hand, a strong repression of eps genes was observed in H. seropedicae cells adhered to maize roots. Our data suggest that H. seropedicae EPS is a structural component of mature biofilms, but this development stage of biofilm is not achieved during plant colonization.
Conflict of interest statement
Figures



Similar articles
-
Maize root lectins mediate the interaction with Herbaspirillum seropedicae via N-acetyl glucosamine residues of lipopolysaccharides.PLoS One. 2013 Oct 9;8(10):e77001. doi: 10.1371/journal.pone.0077001. eCollection 2013. PLoS One. 2013. PMID: 24130823 Free PMC article.
-
Real-time PCR quantification of the plant growth promoting bacteria Herbaspirillum seropedicae strain SmR1 in maize roots.Mol Biotechnol. 2014 Jul;56(7):660-70. doi: 10.1007/s12033-014-9742-4. Mol Biotechnol. 2014. PMID: 24563376
-
Herbaspirillum seropedicae rfbB and rfbC genes are required for maize colonization.Environ Microbiol. 2010 Aug;12(8):2233-44. doi: 10.1111/j.1462-2920.2010.02187.x. Epub 2010 Mar 7. Environ Microbiol. 2010. PMID: 21966916
-
Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance.J Basic Microbiol. 2018 Dec;58(12):1009-1022. doi: 10.1002/jobm.201800309. Epub 2018 Sep 5. J Basic Microbiol. 2018. PMID: 30183106 Review.
-
Bacterial Exopolysaccharides: Insight into Their Role in Plant Abiotic Stress Tolerance.J Microbiol Biotechnol. 2021 Aug 28;31(8):1045-1059. doi: 10.4014/jmb.2105.05009. J Microbiol Biotechnol. 2021. PMID: 34226402 Free PMC article. Review.
Cited by
-
Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress.Front Microbiol. 2020 Aug 20;11:1952. doi: 10.3389/fmicb.2020.01952. eCollection 2020. Front Microbiol. 2020. PMID: 32973708 Free PMC article. Review.
-
Transcriptome profiling of a Rhizobium leguminosarum bv. trifolii rosR mutant reveals the role of the transcriptional regulator RosR in motility, synthesis of cell-surface components, and other cellular processes.BMC Genomics. 2015 Dec 29;16:1111. doi: 10.1186/s12864-015-2332-4. BMC Genomics. 2015. PMID: 26715155 Free PMC article.
-
Differential exopolysaccharide production and composition by Herbaspirillum strains from diverse ecological environments.Arch Microbiol. 2021 Sep;203(7):3883-3892. doi: 10.1007/s00203-021-02371-x. Epub 2021 May 19. Arch Microbiol. 2021. PMID: 34009446
-
Comparative genomic analyses of transport proteins encoded within the genomes of Leptospira species.Microb Pathog. 2015 Nov;88:52-64. doi: 10.1016/j.micpath.2015.07.019. Epub 2015 Aug 3. Microb Pathog. 2015. PMID: 26247102 Free PMC article.
-
Comparative analyses of transport proteins encoded within the genomes of Leptospira species.Microb Pathog. 2016 Sep;98:118-31. doi: 10.1016/j.micpath.2016.06.013. Epub 2016 Jun 11. Microb Pathog. 2016. PMID: 27296707 Free PMC article.
References
-
- Monteiro RA, Balsanelli E, Wassen R, Marin AM, Brusamarello-Santos LCC, et al. (2012) Herbaspirillum-plant interactions: microscopical, histological and molecular aspects. Plant Soil 356: 175–196.
-
- Sutherland IW (1980) Biosynthesis of microbial exopolysaccharides. Ann Rev Microbiol 34: 79–150. - PubMed
-
- Flemming HC, Wingender J (2001) Relevance of microbial extracellular polymeric substances (EPSs)–Part I: structural and ecological aspects. Water Science Technol 43: 1–8. - PubMed
-
- Costerton JW, Lawandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Ann Rev Microbiol 49: 711–745. - PubMed
-
- Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56: 187–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

