Induced pluripotent stem cell therapy ameliorates hyperoxia-augmented ventilator-induced lung injury through suppressing the Src pathway
- PMID: 25310015
- PMCID: PMC4195701
- DOI: 10.1371/journal.pone.0109953
Induced pluripotent stem cell therapy ameliorates hyperoxia-augmented ventilator-induced lung injury through suppressing the Src pathway
Abstract
Background: High tidal volume (VT) mechanical ventilation (MV) can induce the recruitment of neutrophils, release of inflammatory cytokines and free radicals, and disruption of alveolar epithelial and endothelial barriers. It is proposed to be the triggering factor that initiates ventilator-induced lung injury (VILI) and concomitant hyperoxia further aggravates the progression of VILI. The Src protein tyrosine kinase (PTK) family is one of the most critical families to intracellular signal transduction related to acute inflammatory responses. The anti-inflammatory abilities of induced pluripotent stem cells (iPSCs) have been shown to improve acute lung injuries (ALIs); however, the mechanisms regulating the interactions between MV, hyperoxia, and iPSCs have not been fully elucidated. In this study, we hypothesize that Src PTK plays a critical role in the regulation of oxidants and inflammation-induced VILI during hyperoxia. iPSC therapy can ameliorate acute hyperoxic VILI by suppressing the Src pathway.
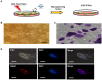
Methods: Male C57BL/6 mice, either wild-type or Src-deficient, aged between 2 and 3 months were exposed to high VT (30 mL/kg) ventilation with or without hyperoxia for 1 to 4 h after the administration of Oct4/Sox2/Parp1 iPSCs at a dose of 5×10(7) cells/kg of mouse. Nonventilated mice were used for the control groups.
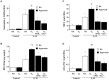
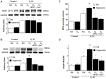
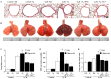
Results: High VT ventilation during hyperoxia further aggravated VILI, as demonstrated by the increases in microvascular permeability, neutrophil infiltration, macrophage inflammatory protein-2 (MIP-2) and plasminogen activator inhibitor-1 (PAI-1) production, Src activation, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, and malaldehyde (MDA) level. Administering iPSCs attenuated ALI induced by MV during hyperoxia, which benefited from the suppression of Src activation, oxidative stress, acute inflammation, and apoptosis, as indicated by the Src-deficient mice.
Conclusion: The data suggest that iPSC-based therapy is capable of partially suppressing acute inflammatory and oxidant responses that occur during hyperoxia-augmented VILI through the inhibition of Src-dependent signaling pathway.
Conflict of interest statement
Figures


Similar articles
-
Activation of Src-dependent Smad3 signaling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury.Respir Res. 2015 Sep 16;16(1):112. doi: 10.1186/s12931-015-0275-6. Respir Res. 2015. PMID: 26377087 Free PMC article.
-
Inhibition of Src and forkhead box O1 signaling by induced pluripotent stem-cell therapy attenuates hyperoxia-augmented ventilator-induced diaphragm dysfunction.Transl Res. 2016 Jul;173:131-147.e1. doi: 10.1016/j.trsl.2016.03.011. Epub 2016 Mar 22. Transl Res. 2016. PMID: 27055225
-
Suppressing NF-κB and NKRF Pathways by Induced Pluripotent Stem Cell Therapy in Mice with Ventilator-Induced Lung Injury.PLoS One. 2013 Jun 26;8(6):e66760. doi: 10.1371/journal.pone.0066760. Print 2013. PLoS One. 2013. PMID: 23840526 Free PMC article.
-
Molecular mechanisms underlying hyperoxia acute lung injury.Respir Med. 2016 Oct;119:23-28. doi: 10.1016/j.rmed.2016.08.010. Epub 2016 Aug 21. Respir Med. 2016. PMID: 27692143 Review.
-
Endothelial cell signaling and ventilator-induced lung injury: molecular mechanisms, genomic analyses, and therapeutic targets.Am J Physiol Lung Cell Mol Physiol. 2017 Apr 1;312(4):L452-L476. doi: 10.1152/ajplung.00231.2016. Epub 2016 Dec 15. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27979857 Free PMC article. Review.
Cited by
-
Activation of Src-dependent Smad3 signaling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury.Respir Res. 2015 Sep 16;16(1):112. doi: 10.1186/s12931-015-0275-6. Respir Res. 2015. PMID: 26377087 Free PMC article.
-
Effectiveness of extracellular vesicles derived from hiPSCs in repairing hyperoxia-induced injury in a fetal murine lung explant model.Stem Cell Res Ther. 2024 Mar 14;15(1):80. doi: 10.1186/s13287-024-03687-3. Stem Cell Res Ther. 2024. PMID: 38486338 Free PMC article.
-
Ventilator-induced diaphragm dysfunction in critical illness.Exp Biol Med (Maywood). 2018 Dec;243(17-18):1329-1337. doi: 10.1177/1535370218811950. Epub 2018 Nov 19. Exp Biol Med (Maywood). 2018. PMID: 30453774 Free PMC article. Review.
-
Human induced pluripotent stem cells ameliorate hyperoxia-induced lung injury in a mouse model.Am J Transl Res. 2020 Jan 15;12(1):292-307. eCollection 2020. Am J Transl Res. 2020. PMID: 32051754 Free PMC article.
-
Cardiopulmonary bypass increases pulmonary microvascular permeability through the Src kinase pathway: Involvement of caveolin-1 and vascular endothelial cadherin.Mol Med Rep. 2016 Mar;13(3):2918-24. doi: 10.3892/mmr.2016.4831. Epub 2016 Jan 29. Mol Med Rep. 2016. PMID: 26847917 Free PMC article.
References
-
- ARDSNet (2000) The acute respiratory distress syndrome network, ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308. - PubMed
-
- Abraham E (2003) Neutrophils and acute lung injury. Crit Care Med 31: S195–199. - PubMed
-
- Held HD, Boettcher S, Hamann L, Uhlig S (2001) Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-kappa B and is blocked by steroids. Am J Respir Crit Care Med 163: 711–716. - PubMed
-
- Ricard JD, Dreyfuss D, Saumon G (2003) Ventilator-induced lung injury. Eur Respir J 22: 2 s-9s. - PubMed
-
- Tremblay LN, Slutsky AS (2006) Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32: 24–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

