Immunogenicity evaluation of a rationally designed polytope construct encoding HLA-A*0201 restricted epitopes derived from Leishmania major related proteins in HLA-A2/DR1 transgenic mice: steps toward polytope vaccine
- PMID: 25310094
- PMCID: PMC4195657
- DOI: 10.1371/journal.pone.0108848
Immunogenicity evaluation of a rationally designed polytope construct encoding HLA-A*0201 restricted epitopes derived from Leishmania major related proteins in HLA-A2/DR1 transgenic mice: steps toward polytope vaccine
Abstract
Background: There are several reports demonstrating the role of CD8 T cells against Leishmania species. Therefore peptide vaccine might represent an effective approach to control the infection. We developed a rational polytope-DNA construct encoding immunogenic HLA-A2 restricted peptides and validated the processing and presentation of encoded epitopes in a preclinical mouse model humanized for the MHC-class-I and II.
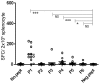
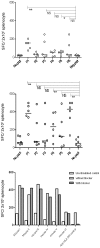
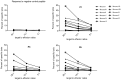
Methods and findings: HLA-A*0201 restricted epitopes from LPG-3, LmSTI-1, CPB and CPC along with H-2Kd restricted peptides, were lined-up together as a polytope string in a DNA construct. Polytope string was rationally designed by harnessing advantages of ubiquitin, spacers and HLA-DR restricted Th1 epitope. Endotoxin free pcDNA plasmid expressing the polytope was inoculated into humanized HLA-DRB1*0101/HLA-A*0201 transgenic mice intramuscularly 4 days after Cardiotoxin priming followed by 2 boosters at one week interval. Mice were sacrificed 10 days after the last booster, and splenocytes were subjected to ex-vivo and in-vitro evaluation of specific IFN-γ production and in-vitro cytotoxicity against individual peptides by ELISpot and standard chromium-51 (51Cr) release assay respectively. 4 H-2Kd and 5 HLA-A*0201 restricted peptides were able to induce specific CD8 T cell responses in BALB/C and HLA-A2/DR1 mice respectively. IFN-γ and cytolytic activity together discriminated LPG-3-P1 as dominant, LmSTI-1-P3 and LmSTI-1-P6 as subdominant with both cytolytic activity and IFN-γ production, LmSTI-1-P4 and LPG-3-P5 as subdominant with only IFN-γ production potential.
Conclusions: Here we described a new DNA-polytope construct for Leishmania vaccination encompassing immunogenic HLA-A2 restricted peptides. Immunogenicity evaluation in HLA-transgenic model confirmed CD8 T cell induction with expected affinities and avidities showing almost efficient processing and presentation of the peptides in relevant preclinical model. Further evaluation will determine the efficacy of this polytope construct protecting against infectious challenge of Leishmania. Fortunately HLA transgenic mice are promising preclinical models helping to speed up immunogenicity analysis in a human related mouse model.
Conflict of interest statement
Figures

Similar articles
-
In silico analysis of six known Leishmania major antigens and in vitro evaluation of specific epitopes eliciting HLA-A2 restricted CD8 T cell response.PLoS Negl Trop Dis. 2011 Sep;5(9):e1295. doi: 10.1371/journal.pntd.0001295. Epub 2011 Sep 6. PLoS Negl Trop Dis. 2011. PMID: 21909442 Free PMC article.
-
Vaccine potential of HLA-A2 epitopes from Leishmania Cysteine Protease Type III (CPC).Parasite Immunol. 2017 Sep;39(9). doi: 10.1111/pim.12451. Epub 2017 Jul 28. Parasite Immunol. 2017. PMID: 28665520
-
Design of multi-epitope peptides containing HLA class-I and class-II-restricted epitopes derived from immunogenic Leishmania proteins, and evaluation of CD4+ and CD8+ T cell responses induced in cured cutaneous leishmaniasis subjects.PLoS Negl Trop Dis. 2020 Mar 16;14(3):e0008093. doi: 10.1371/journal.pntd.0008093. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32176691 Free PMC article.
-
Human genetics of leishmania infections.Hum Genet. 2020 Jun;139(6-7):813-819. doi: 10.1007/s00439-020-02130-w. Epub 2020 Feb 13. Hum Genet. 2020. PMID: 32055998 Free PMC article. Review.
-
Leishmaniac Quest for Developing a Novel Vaccine Platform. Is a Roadmap for Its Advances Provided by the Mad Dash to Produce Vaccines for COVID-19?Vaccines (Basel). 2022 Feb 7;10(2):248. doi: 10.3390/vaccines10020248. Vaccines (Basel). 2022. PMID: 35214706 Free PMC article. Review.
Cited by
-
A Leishmania-specific hypothetical protein expressed in both promastigote and amastigote stages of Leishmania infantum employed for the serodiagnosis of, and as a vaccine candidate against, visceral leishmaniasis.Parasit Vectors. 2015 Jul 11;8:363. doi: 10.1186/s13071-015-0964-5. Parasit Vectors. 2015. PMID: 26160291 Free PMC article.
-
Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis.Vaccines (Basel). 2020 May 27;8(2):252. doi: 10.3390/vaccines8020252. Vaccines (Basel). 2020. PMID: 32471081 Free PMC article.
-
A Chimera Containing CD4+ and CD8+ T-Cell Epitopes of the Leishmania donovani Nucleoside Hydrolase (NH36) Optimizes Cross-Protection against Leishmania amazonesis Infection.Front Immunol. 2017 Feb 23;8:100. doi: 10.3389/fimmu.2017.00100. eCollection 2017. Front Immunol. 2017. PMID: 28280494 Free PMC article.
-
How Can Elispot Add Information to Improve Knowledge on Tropical Diseases?Cells. 2017 Sep 29;6(4):31. doi: 10.3390/cells6040031. Cells. 2017. PMID: 28961208 Free PMC article. Review.
-
New Approaches to the Prevention of Visceral Leishmaniasis: A Review of Recent Patents of Potential Candidates for a Chimeric Protein Vaccine.Vaccines (Basel). 2024 Mar 5;12(3):271. doi: 10.3390/vaccines12030271. Vaccines (Basel). 2024. PMID: 38543905 Free PMC article. Review.
References
-
- Organization WH (2010) Control of the leishmaniases. World Health Organization technical report series: xii - PubMed
-
- Croft SL, Olliaro P (2011) Leishmaniasis chemotherapy–challenges and opportunities. Clin Microbiol Infect 17: 1478–1483. - PubMed
-
- Kishore K, Kumar V, Kesari S, Dinesh DS, Kumar AJ, et al. (2006) Vector control in leishmaniasis. Indian J Med Res 123: 467–472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

