Angiogenic potential of vitreous from Proliferative Diabetic Retinopathy and Eales' Disease patients
- PMID: 25310689
- PMCID: PMC4195571
- DOI: 10.1371/journal.pone.0107551
Angiogenic potential of vitreous from Proliferative Diabetic Retinopathy and Eales' Disease patients
Abstract
Purpose: Proliferative Diabetic Retinopathy (PDR) and Eales' Disease (ED) have different aetiologies although they share certain common clinical symptoms including pre-retinal neovascularization. Since there is a need to understand if the shared end-stage angiogenic pathology of PDR and ED is driven by common stimulating factors, we have studied the cytokines contained in vitreous from both patient groups and analyzed the angiogenic potential of these samples in vitro.
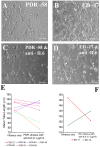
Material and methods: Vitreous samples from patients with PDR (n = 13) and ED (n = 5) were quantified for various cytokines using a cytokine biochip array and sandwich ELISA. An additional group of patients (n = 5) with macular hole (MH) was also studied for comparison. To determine the angiogenic potential of these vitreous samples, they were analyzed for their ability to induce tubulogenesis in human microvascular endothelial cells. Further, the effect of anti-VEGF (Ranibizumab) and anti-IL-6 antibodies were studied on vitreous-mediated vascular tube formation.
Results: Elevated levels of IL-6, IL-8, MCP-1 and VEGF were observed in vitreous of both PDR and ED when compared to MH. PDR and ED vitreous induced greater levels of endothelial cell tube formation compared to controls without vitreous (P<0.05). When VEGF in vitreous was neutralized by clinically-relevant concentrations of Ranibizumab, tube length was reduced significantly in 5 of 6 PDR and 3 of 5 ED samples. Moreover, when treated with IL-6 neutralizing antibody, apparent reduction (71.4%) was observed in PDR vitreous samples.
Conclusions: We have demonstrated that vitreous specimens from PDR and ED patients share common elevations of pro-inflammatory and pro-angiogenic cytokines. This suggests that common cytokine profiles link these two conditions.
Conflict of interest statement
Figures




References
-
- Das T, Biswas J, Kumar A, Nagpal PN, Namperumalsamy P, et al. (1994) Eales' disease. Indian J Ophthalmol 42: 3–18. - PubMed
-
- Murthy KR, Abraham C, Baig SM,Badrinath SS (1977) Eales' disease. Proc All Ind Ophthalmol. pp. 323.
-
- Atmaca LS, Batioglu F, Atmaca Sonmez P (2002) A long-term follow-up of Eales' disease. Ocul Immunol Inflamm 10: 213–221. - PubMed
-
- Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, et al. (2008) Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales' disease. Retina 28: 817–824. - PubMed
-
- Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391–400. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

