Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells
- PMID: 25310984
- PMCID: PMC4266564
- DOI: 10.1016/j.celrep.2014.09.013
Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells
Abstract
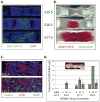
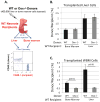
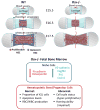
Hematopoietic stem cells (HSCs) reside within a specialized niche where interactions with vasculature, osteoblasts, and stromal components regulate their self-renewal and differentiation. Little is known about bone marrow niche formation or the role of its cellular components in HSC development; therefore, we established the timing of murine fetal long bone vascularization and ossification relative to the onset of HSC activity. Adult-repopulating HSCs emerged at embryonic day 16.5 (E16.5), coincident with marrow vascularization, and were contained within the c-Kit(+)Sca-1(+)Lin(-) (KSL) population. We used Osterix-null (Osx(-/-)) mice that form vascularized marrow but lack osteolineage cells to dissect the role(s) of these cellular components in HSC development. Osx(-/-) fetal bone marrow cells formed multilineage colonies in vitro but were hyperproliferative and failed to home to and/or engraft transplant recipients. Thus, in developing bone marrow, the vasculature can sustain multilineage progenitors, but interactions with osteolineage cells are needed to regulate long-term HSC proliferation and potential.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

