Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031
- PMID: 25311218
- PMCID: PMC4220044
- DOI: 10.1200/JCO.2013.52.5410
Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031
Abstract
Purpose: The Children's Oncology Group study AHOD0031, a randomized phase III study, was designed to evaluate the role of early chemotherapy response in tailoring subsequent therapy in pediatric intermediate-risk Hodgkin lymphoma. To avoid treatment-associated risks that compromise long-term health and to maintain high cure rates, dose-intensive chemotherapy with limited cumulative doses was used.
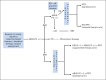
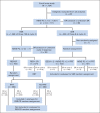
Patients and methods: Patients received two cycles of doxorubicin, bleomycin, vincristine, etoposide, cyclophosphamide, and prednisone (ABVE-PC) followed by response evaluation. Rapid early responders (RERs) received two additional ABVE-PC cycles, followed by complete response (CR) evaluation. RERs with CR were randomly assigned to involved-field radiotherapy (IFRT) or no additional therapy; RERs with less than CR were nonrandomly assigned to IFRT. Slow early responders (SERs) were randomly assigned to receive two additional ABVE-PC cycles with or without two cycles of dexamethasone, etoposide, cisplatin, and cytarabine (DECA). All SERs were assigned to receive IFRT.
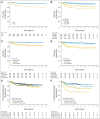
Results: Among 1,712 eligible patients, 4-year event-free survival (EFS) was 85.0%: 86.9% for RERs and 77.4% for SERs (P < .001). Four-year overall survival was 97.8%: 98.5% for RERs and 95.3% for SERs (P < .001). Four-year EFS was 87.9% versus 84.3% (P = .11) for RERs with CR who were randomly assigned to IFRT versus no IFRT, and 86.7% versus 87.3% (P = .87) for RERs with positron emission tomography (PET) -negative results at response assessment. Four-year EFS was 79.3% versus 75.2% (P = .11) for SERs who were randomly assigned to DECA versus no DECA, and 70.7% versus 54.6% (P = .05) for SERs with PET-positive results at response assessment.
Conclusion: This trial demonstrated that early response assessment supported therapeutic titration (omitting radiotherapy in RERs with CR; augmenting chemotherapy in SERs with PET-positive disease). Strategies directed toward improved response assessment and risk stratification may enhance tailoring of treatment to patient characteristics and response.
Trial registration: ClinicalTrials.gov NCT00025259.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; 2012. http://seer.cancer.gov/csr/1975_2009_pops09/
-
- Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

