Apo, Zn2+-bound and Mn2+-bound structures reveal ligand-binding properties of SitA from the pathogen Staphylococcus pseudintermedius
- PMID: 25311310
- PMCID: PMC4242081
- DOI: 10.1042/BSR20140088
Apo, Zn2+-bound and Mn2+-bound structures reveal ligand-binding properties of SitA from the pathogen Staphylococcus pseudintermedius
Abstract
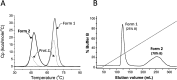
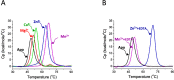
The Gram-positive bacterium Staphylococcus pseudintermedius is a leading cause of canine bacterial pyoderma, resulting in worldwide morbidity in dogs. S. pseudintermedius also causes life-threatening human infections. Furthermore, methicillin-resistant S. pseudintermedius is emerging, resembling the human health threat of methicillin-resistant Staphylococcus aureus. Therefore it is increasingly important to characterize targets for intervention strategies to counteract S. pseudintermedius infections. Here we used biophysical methods, mutagenesis, and X-ray crystallography, to define the ligand-binding properties and structure of SitA, an S. pseudintermedius surface lipoprotein. SitA was strongly and specifically stabilized by Mn2+ and Zn2+ ions. Crystal structures of SitA complexed with Mn2+ and Zn2+ revealed a canonical class III solute-binding protein with the metal cation bound in a cavity between N- and C-terminal lobes. Unexpectedly, one crystal contained both apo- and holo-forms of SitA, revealing a large side-chain reorientation of His64, and associated structural differences accompanying ligand binding. Such conformational changes may regulate fruitful engagement of the cognate ABC (ATP-binding cassette) transporter system (SitBC) required for metal uptake. These results provide the first detailed characterization and mechanistic insights for a potential therapeutic target of the major canine pathogen S. pseudintermedius, and also shed light on homologous structures in related staphylococcal pathogens afflicting humans.
Figures
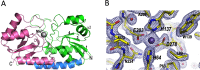
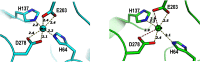



References
-
- Bannoehr J., Ben Zakour N. L., Waller A. S., Guardabassi L., Thoday K. L., van den Broek A. H., Fitzgerald J. R. Population genetic structure of the Staphylococcus intermedius group: insights into agr diversification and the emergence of methicillin-resistant strains. J. Bacteriol. 2007;189:8685–8692. doi: 10.1128/JB.01150-07. - DOI - PMC - PubMed
-
- Hill P. B., Lo A., Eden C. A., Huntley S., Morey V., Ramsey S., Richardson C., Smith D. J., Sutton C., Taylor M. D., et al. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. Vet. Rec. 2006;158:533–539. doi: 10.1136/vr.158.16.533. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

