Oxidative Glial Cell Damage Associated with White Matter Lesions in the Aging Human Brain
- PMID: 25311358
- PMCID: PMC4861214
- DOI: 10.1111/bpa.12216
Oxidative Glial Cell Damage Associated with White Matter Lesions in the Aging Human Brain
Abstract
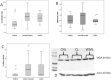
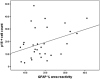
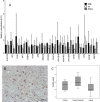
White matter lesions (WML) are common in brain aging and are associated with dementia. We aimed to investigate whether oxidative DNA damage and occur in WML and in apparently normal white matter in cases with lesions. Tissue from WML and control white matter from brains with lesions (controls lesional) and without lesions (controls non-lesional) were obtained, using post-mortem magnetic resonance imaging-guided sampling, from the Medical Research Council Cognitive Function and Ageing Study. Oxidative damage was assessed by immunohistochemistry to 8-hydroxy-2'-deoxoguanosine (8-OHdG) and Western blotting for malondialdehyde. DNA response was assessed by phosphorylated histone H2AX (γH2AX), p53, senescence markers and by quantitative Reverse transcription polymerase chain reaction (RT-PCR) panel for candidate DNA damage-associated genes. 8-OHdG was expressed in glia and endothelium, with increased expression in both WML and controls lesional compared with controls non-lesional (P < 0.001). γH2Ax showed a similar, although attenuated difference among groups (P = 0.03). Expression of senescence-associated β-galactosidase and p16 suggested induction of senescence mechanisms in glia. Oxidative DNA damage and a DNA damage response are features of WML pathogenesis and suggest candidate mechanisms for glial dysfunction. Their expression in apparently normal white matter in cases with WML suggests that white matter dysfunction is not restricted to lesions. The role of this field-effect lesion pathogenesis and cognitive impairment are areas to be defined.
Keywords: DNA damage; dementia; ischemic white matter; white matter disease; white matter lesions.
© 2014 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Figures




References
-
- Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H (1997) Alterations in glia and axons in the brains of Binswangers disease patients. Stroke 28:1423–1429. - PubMed
-
- Breteler M, Van Swieten J, Bots M, Grobbee D, Claus J, Van Den Hout J et al (1994) Cerebral white matter lesions, vascular risk factors, and cognitive function in a population‐based study: the Rotterdam Study. Neurology 44:1246–1252. - PubMed
-
- Chalmers K, Wilcock G, Love S (2005) Contributors to white matter damage in the frontal lobe in Alzheimer's disease. Neuropathol Appl Neurobiol 31:623–631. - PubMed
-
- Clerici F, Caracciolo B, Cova I, Fusari I, Maggiore L, Galimberti D et al (2012) Does vascular burden contribute to the progression of mild cognitive impairment to dementia? Dement Geriatr Cogn Disord 34:235–243. - PubMed
Publication types
MeSH terms
Grants and funding
- MRC U.1052.00.0013/MRC_/Medical Research Council/United Kingdom
- MR/L016451/1/MRC_/Medical Research Council/United Kingdom
- MC_U105292687/MRC_/Medical Research Council/United Kingdom
- BB/K006711/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0900652/MRC_/Medical Research Council/United Kingdom
- G0601022/MRC_/Medical Research Council/United Kingdom
- G0502157/MRC_/Medical Research Council/United Kingdom
- G0400074/MRC_/Medical Research Council/United Kingdom
- MRC/G9901400/MRC_/Medical Research Council/United Kingdom
- G9901400/MRC_/Medical Research Council/United Kingdom
- MR/L022656/1/MRC_/Medical Research Council/United Kingdom
- NF-SI-0611-10084/DH_/Department of Health/United Kingdom
- G1100540/MRC_/Medical Research Council/United Kingdom
- MR/J004308/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

