Calmodulin enhances ribbon replenishment and shapes filtering of synaptic transmission by cone photoreceptors
- PMID: 25311636
- PMCID: PMC4210432
- DOI: 10.1085/jgp.201411229
Calmodulin enhances ribbon replenishment and shapes filtering of synaptic transmission by cone photoreceptors
Abstract
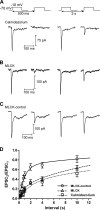
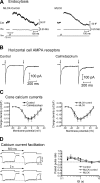
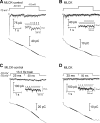
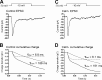
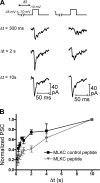
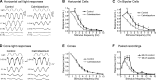
At the first synapse in the vertebrate visual pathway, light-evoked changes in photoreceptor membrane potential alter the rate of glutamate release onto second-order retinal neurons. This process depends on the synaptic ribbon, a specialized structure found at various sensory synapses, to provide a supply of primed vesicles for release. Calcium (Ca(2+)) accelerates the replenishment of vesicles at cone ribbon synapses, but the mechanisms underlying this acceleration and its functional implications for vision are unknown. We studied vesicle replenishment using paired whole-cell recordings of cones and postsynaptic neurons in tiger salamander retinas and found that it involves two kinetic mechanisms, the faster of which was diminished by calmodulin (CaM) inhibitors. We developed an analytical model that can be applied to both conventional and ribbon synapses and showed that vesicle resupply is limited by a simple time constant, τ = 1/(Dρδs), where D is the vesicle diffusion coefficient, δ is the vesicle diameter, ρ is the vesicle density, and s is the probability of vesicle attachment. The combination of electrophysiological measurements, modeling, and total internal reflection fluorescence microscopy of single synaptic vesicles suggested that CaM speeds replenishment by enhancing vesicle attachment to the ribbon. Using electroretinogram and whole-cell recordings of light responses, we found that enhanced replenishment improves the ability of cone synapses to signal darkness after brief flashes of light and enhances the amplitude of responses to higher-frequency stimuli. By accelerating the resupply of vesicles to the ribbon, CaM extends the temporal range of synaptic transmission, allowing cones to transmit higher-frequency visual information to downstream neurons. Thus, the ability of the visual system to encode time-varying stimuli is shaped by the dynamics of vesicle replenishment at photoreceptor synaptic ribbons.
© 2014 Van Hook et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

