Intraclonal diversity in follicular lymphoma analyzed by quantitative ultradeep sequencing of noncoding regions
- PMID: 25311808
- PMCID: PMC4225181
- DOI: 10.4049/jimmunol.1401699
Intraclonal diversity in follicular lymphoma analyzed by quantitative ultradeep sequencing of noncoding regions
Abstract
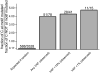
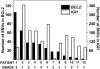
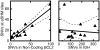
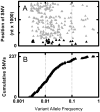
Cancers are characterized by genomic instability, and the resulting intraclonal diversity is a prerequisite for tumor evolution. Therefore, metrics of tumor heterogeneity may prove to be clinically meaningful. Intraclonal heterogeneity in follicular lymphoma (FL) is apparent from studies of somatic hypermutation (SHM) caused by activation-induced deaminase (AID) in IGH. Aberrant SHM (aSHM), defined as AID activity outside of the IG loci, predominantly targets noncoding regions causing numerous "passenger" mutations, but it has the potential to generate rare significant "driver" mutations. The quantitative relationship between SHM and aSHM has not been defined. To measure SHM and aSHM, ultradeep sequencing (>20,000-fold coverage) was performed on IGH (~1650 nt) and nine other noncoding regions potentially targeted by AID (combined 9411 nt), including the 5' untranslated region of BCL2. Single-nucleotide variants (SNVs) were found in 12/12 FL specimens (median 136 SHMs and 53 aSHMs). The aSHM SNVs were associated with AID motifs (p < 0.0001). The number of SNVs at BCL2 varied widely among specimens and correlated with the number of SNVs at eight other potential aSHM sites. In contrast, SHM at IGH was not predictive of aSHM. Tumor heterogeneity is apparent from SNVs at low variant allele frequencies; the relative number of SNVs with variable allele frequency < 5% varied with clinical grade, indicating that tumor heterogeneity based on aSHM reflects a clinically meaningful parameter. These data suggest that genome-wide aSHM may be estimated from aSHM of BCL2 but not SHM of IGH. The results demonstrate a practical approach to the quantification of intratumoral genetic heterogeneity for clinical specimens.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

References
-
- Casulo C, Burack WR, Friedberg JW. Transformed Lymphoma: A Therapeutic Challenge. Blood 2014 - PubMed
-
- Bernstein SH, Burack WR. The incidence, natural history, biology, and treatment of transformed lymphomas. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2009:532–541. - PubMed
-
- Limpens J, Stad R, Vos C, de Vlaam C, de Jong D, van Ommen GJ, Schuuring E, Kluin PM. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood. 1995;85:2528–2536. - PubMed
-
- Roulland S, Faroudi M, Mamessier E, Sungalee S, Salles G, Nadel B. Early steps of follicular lymphoma pathogenesis. Advances in immunology. 2011;111:1–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

