Clinical and molecular characteristics of childhood-onset Stargardt disease
- PMID: 25312043
- PMCID: PMC4459618
- DOI: 10.1016/j.ophtha.2014.08.012
Clinical and molecular characteristics of childhood-onset Stargardt disease
Abstract
Purpose: To describe the clinical and molecular characteristics of patients with childhood-onset Stargardt disease (STGD).
Design: Retrospective case series.
Participants: Forty-two patients who were diagnosed with STGD in childhood at a single institution between January 2001 and January 2012.
Methods: A detailed history and a comprehensive ophthalmic examination were undertaken, including color fundus photography, autofluorescence imaging, spectral-domain optical coherence tomography (SD-OCT), and pattern and full-field electroretinograms. The entire coding region and splice sites of ABCA4 were screened using a next-generation, sequencing-based strategy. The molecular genetic findings of childhood-onset STGD patients were compared with those of adult-onset patients.
Main outcome measures: Clinical, imaging, electrophysiologic, and molecular genetic findings.
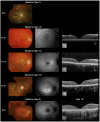
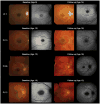
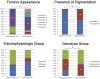
Results: The median ages of onset and the median age at baseline examination were 8.5 (range, 3-16) and 12.0 years (range, 7-16), respectively. The median baseline logarithm of the minimum angle of resolution visual acuity was 0.74. At baseline, 26 of 39 patients (67%) with available photographs had macular atrophy with macular/peripheral flecks; 11 (28%) had macular atrophy without flecks; 1 (2.5%) had numerous flecks without macular atrophy; and 1 (2.5%) had a normal fundus appearance. Flecks were not identified at baseline in 12 patients (31%). SD-OCT detected foveal outer retinal disruption in all 21 patients with available images. Electrophysiologic assessment demonstrated retinal dysfunction confined to the macula in 9 patients (36%), macular and generalized cone dysfunction in 1 subject (4%), and macular and generalized cone and rod dysfunction in 15 individuals (60%). At least 1 disease-causing ABCA4 variant was identified in 38 patients (90%), including 13 novel variants; ≥2 variants were identified in 34 patients (81%). Patients with childhood-onset STGD more frequently harbored 2 deleterious variants (18% vs 5%) compared with patients with adult-onset STGD.
Conclusions: Childhood-onset STGD is associated with severe visual loss, early morphologic changes, and often generalized retinal dysfunction, despite often having less severe fundus abnormalities on examination. One third of children do not have flecks at presentation. The relatively high proportion of deleterious ABCA4 variants supports the hypothesis that earlier onset disease is often owing to more severe variants in ABCA4 than those found in adult-onset disease.
Copyright © 2015 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–46. - PubMed
-
- Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976;94:2061–7. - PubMed
-
- Fujinami K, Lois N, Davidson AE, et al. A longitudinal study of Stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correlations. Am J Ophthalmol. 2013;155:1075–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

