The establishment of sub-strain specific WHO Reference Reagents for BCG vaccine
- PMID: 25312272
- PMCID: PMC4226838
- DOI: 10.1016/j.vaccine.2014.09.065
The establishment of sub-strain specific WHO Reference Reagents for BCG vaccine
Abstract
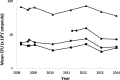
As the latest addition to the sub-strain specific WHO Reference Reagents of BCG vaccine, an international collaborative study was completed to evaluate the suitability of a candidate BCG Moreau-RJ sub-strain as a WHO Reference Reagent of BCG vaccine. This follows the recent replacement of the WHO 1st International Reference Preparation for BCG vaccine, by three sub-strain specific WHO Reference Reagents of BCG vaccine (Danish 1331, Tokyo 172-1 and Russian BCG-I) in order to complete the coverage of most predominant sub-strains used for BCG vaccine production and distribution for use worldwide. The study used cultural viable count and modified ATP assays to quantify the preparation and multiplex PCR to confirm the identity of the sub-strain. The establishment of this WHO Reference Reagent of BCG vaccine of Moreau-RJ sub-strain was approved by the WHO Expert Committee on Biological Standardization meeting in October 2012. This preparation is available for distribution by NIBSC-MHRA, UK. The data from real-time stability monitoring demonstrated that these Reference Reagents of BCG vaccine are very stable in storage condition at -20°C. They serve as the valuable source of BCG Reference Reagents for use as comparators (1) for viability assays (such as cultural viable count and modified ATP assays); (2) for in vivo assays (such as the absence of virulent mycobacteria, dermal reactivity and protection assays) in the evaluation of candidate TB vaccines in non-clinical models; (3) for identity assays using molecular biology techniques.
Keywords: ATP; BCG; Reference reagent; Stability; Vaccine; Viability; WHO.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
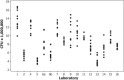


 , (b) Tokyo 172-1
, (b) Tokyo 172-1  , (c) Russian BCG-I
, (c) Russian BCG-I  , and (d) Moreau-RJ
, and (d) Moreau-RJ  , using the cultural viable count assay.
, using the cultural viable count assay.References
-
- Corbel M.J., Fruth U., Griffiths E., Knezevic I. Report on a WHO consultation on the characterisation of BCG strains, Imperial College, London 15–16 December 2003. Vaccine. 2004;22:2675–2680. - PubMed
-
- Ho M.M., Corbel M.J., Knezevic I., Roumiantzeff M. Report on a WHO consultation on the characterisation of BCG vaccines, WHO, Geneva, Switzerland 8–9 December 2004. Vaccine. 2005;23:5700–5704.
-
- Knezevic I., Corbel M.J. WHO discussion on the improvement of the quality control of BCG vaccines. Pasteur Institute, Paris, France, 7 June 2005. Vaccine. 2006;24:3874–3877. - PubMed
-
- WHO Expert Committee on Biological Standardization . 2011. Recommendations to assure the quality, safety and efficacy of BCG vaccines. World Health Organisation Technical Report Series; No. 979: Annex 3; pp. 137–185.
-
- Directorate for the Quality of Medicines of the Council of Europe (EDQM); Strasbourg, Cedex, France: 2012. European pharmacopoeia; pp. 819–820. BCG vaccine, freeze-dried. 01/2012: 0163.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

