Gαi2-protein-mediated signal transduction: central nervous system molecular mechanism countering the development of sodium-dependent hypertension
- PMID: 25312437
- PMCID: PMC4268057
- DOI: 10.1161/HYPERTENSIONAHA.114.04463
Gαi2-protein-mediated signal transduction: central nervous system molecular mechanism countering the development of sodium-dependent hypertension
Abstract
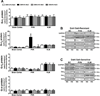
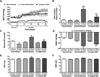
Excess dietary salt intake is an established cause of hypertension. At present, our understanding of the neuropathophysiology of salt-sensitive hypertension is limited by a lack of identification of the central nervous system mechanisms that modulate sympathetic outflow and blood pressure in response to dietary salt intake. We hypothesized that impairment of brain Gαi2-protein-gated signal transduction pathways would result in increased sympathetically mediated renal sodium retention, thus promoting the development of salt-sensitive hypertension. To test this hypothesis, naive or renal denervated Dahl salt-resistant and Dahl salt-sensitive (DSS) rats were assigned to receive a continuous intracerebroventricular control scrambled or a targeted Gαi2-oligodeoxynucleotide infusion, and naive Brown Norway and 8-congenic DSS rats were fed a 21-day normal or high-salt diet. High salt intake did not alter blood pressure, suppressed plasma norepinephrine, and evoked a site-specific increase in hypothalamic paraventricular nucleus Gαi2-protein levels in naive Brown Norway, Dahl salt-resistant, and scrambled oligodeoxynucleotide-infused Dahl salt-resistant but not DSS rats. In Dahl salt-resistant rats, Gαi2 downregulation evoked rapid renal nerve-dependent hypertension, sodium retention, and sympathoexcitation. In DSS rats, Gαi2 downregulation exacerbated salt-sensitive hypertension via a renal nerve-dependent mechanism. Congenic-8 DSS rats exhibited sodium-evoked paraventricular nucleus-specific Gαi2-protein upregulation and attenuated hypertension, sodium retention, and global sympathoexcitation compared with DSS rats. These data demonstrate that paraventricular nucleus Gαi2-protein-gated pathways represent a conserved central molecular pathway mediating sympathoinhibitory renal nerve-dependent responses evoked to maintain sodium homeostasis and a salt-resistant phenotype. Impairment of this mechanism contributes to the development of salt-sensitive hypertension.
Keywords: blood pressure regulation; central G-protein–coupled receptors; renal sympathetic nerves; salt-sensitive hypertension; sympathetic nervous system.
© 2014 American Heart Association, Inc.
Conflict of interest statement
None
Figures




References
-
- Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease—a delicate balance. N Engl J Med. 2013;368:1229–1237. - PubMed
-
- Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. - PubMed
-
- Franco V, Oparil S. Salt Sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Med Coll Nutrit. 2006;25:S247–S255. - PubMed
-
- Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol. 2005;32:426–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

