The impact of abdominal pain on global measures in patients with chronic idiopathic constipation, before and after treatment with linaclotide: a pooled analysis of two randomised, double-blind, placebo-controlled, phase 3 trials
- PMID: 25312449
- PMCID: PMC4278547
- DOI: 10.1111/apt.12985
The impact of abdominal pain on global measures in patients with chronic idiopathic constipation, before and after treatment with linaclotide: a pooled analysis of two randomised, double-blind, placebo-controlled, phase 3 trials
Abstract
Background: Few clinical trials in chronic idiopathic constipation (CIC) patients have evaluated abdominal symptom severity and whether CIC patients with abdominal symptoms respond similarly to patients with limited abdominal symptoms.
Aims: To examine abdominal symptom severity and relationships between symptoms and global measures at baseline; compare linaclotide's effect on symptoms in subpopulations with more or less abdominal pain; and assess relationships between symptom improvement and global measures in these two subpopulations.
Methods: In two phase 3 trials, patients meeting modified Rome II CIC criteria were assigned to linaclotide 145 μg, 290 μg, or placebo once daily. Patients rated abdominal and bowel symptoms daily during 2-week pre-treatment and 12-week treatment periods. Linaclotide's effect on symptoms and global measures [constipation severity, health-related quality of life (HRQOL), treatment satisfaction] and their inter-relationships were assessed in post hoc analyses of abdominal pain subpopulations.
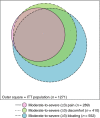
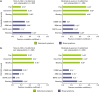
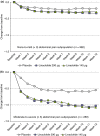
Results: Of 1271 CIC patients, 23%, 32%, and 43% reported moderate-to-severe abdominal pain, discomfort, and bloating, respectively, during baseline. In more-severe abdominal pain patients, abdominal symptoms were more strongly correlated than bowel symptoms with global measures, but in less-severe abdominal pain patients, abdominal and bowel symptoms were similarly correlated with global measures, at baseline and post-treatment. Linaclotide significantly improved all symptoms and global measures in both subpopulations.
Conclusions: When abdominal pain is present in CIC, abdominal and not bowel symptoms may drive patient assessments of constipation severity, HRQOL, and treatment satisfaction. Linaclotide (145 μg and 290 μg) is an effective treatment for both abdominal and bowel symptoms, even in CIC patients with more severe abdominal pain at baseline. (Clinicaltrials.gov: NCT00765882, NCT00730015).
© 2014 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

References
-
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–9. - PubMed
-
- Suares NC, Ford AC. Diagnosis and treatment of irritable bowel syndrome. Discov Med. 2011;11:425–33. - PubMed
-
- Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–8. - PubMed
-
- Wald A, Scarpignato C, Kamm MA, et al. The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther. 2007;26:227–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

