BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages
- PMID: 25312496
- PMCID: PMC4276600
- DOI: 10.1016/j.stem.2014.09.009
BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages
Abstract
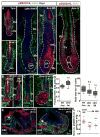
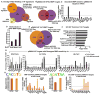
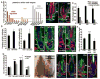
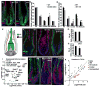
Hair follicle stem cells (HFSCs) and their transit amplifying cell (TAC) progeny sense BMPs at defined stages of the hair cycle to control their proliferation and differentiation. Here, we exploit the distinct spatial and temporal localizations of these cells to selectively ablate BMP signaling in each compartment and examine its functional role. We find that BMP signaling is required for HFSC quiescence and to promote TAC differentiation along different lineages as the hair cycle progresses. We also combine in vivo genome-wide chromatin immunoprecipitation and deep-sequencing, transcriptional profiling, and loss-of-function genetics to define BMP-regulated genes. We show that some pSMAD1/5 targets, like Gata3, function specifically in TAC lineage-progression. Others, like Id1 and Id3, function in both HFSCs and TACs, but in distinct ways. Our study therefore illustrates the complex differential roles that a key signaling pathway can play in regulation of closely related stem/progenitor cells within the context of their overall niche.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Temporal Layering of Signaling Effectors Drives Chromatin Remodeling during Hair Follicle Stem Cell Lineage Progression.Cell Stem Cell. 2018 Mar 1;22(3):398-413.e7. doi: 10.1016/j.stem.2017.12.004. Epub 2018 Jan 11. Cell Stem Cell. 2018. PMID: 29337183 Free PMC article.
-
Smad1 and 5 but not Smad8 establish stem cell quiescence which is critical to transform the premature hair follicle during morphogenesis toward the postnatal state.Stem Cells. 2014 Feb;32(2):534-47. doi: 10.1002/stem.1548. Stem Cells. 2014. PMID: 24023003 Free PMC article.
-
miR-203a-3p promotes loureirin A-induced hair follicle stem cells differentiation by targeting Smad1.Anat Rec (Hoboken). 2021 Mar;304(3):531-540. doi: 10.1002/ar.24480. Epub 2020 Aug 11. Anat Rec (Hoboken). 2021. PMID: 32589363
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
A systematic summary of survival and death signalling during the life of hair follicle stem cells.Stem Cell Res Ther. 2021 Aug 11;12(1):453. doi: 10.1186/s13287-021-02527-y. Stem Cell Res Ther. 2021. PMID: 34380571 Free PMC article. Review.
Cited by
-
Photobiomodulation therapy for hair regeneration: A synergetic activation of β-CATENIN in hair follicle stem cells by ROS and paracrine WNTs.Stem Cell Reports. 2021 Jun 8;16(6):1568-1583. doi: 10.1016/j.stemcr.2021.04.015. Epub 2021 May 20. Stem Cell Reports. 2021. PMID: 34019818 Free PMC article.
-
Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity.Nat Rev Mol Cell Biol. 2023 May;24(5):334-354. doi: 10.1038/s41580-022-00568-6. Epub 2023 Mar 15. Nat Rev Mol Cell Biol. 2023. PMID: 36922629 Free PMC article. Review.
-
[Research progress in regulation of hair growth by dermal adipose tissue].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 May 15;38(5):626-632. doi: 10.7507/1002-1892.202402092. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 38752252 Free PMC article. Review. Chinese.
-
Modelling Human Hair Follicles-Lessons from Animal Models and Beyond.Biology (Basel). 2024 Apr 30;13(5):312. doi: 10.3390/biology13050312. Biology (Basel). 2024. PMID: 38785794 Free PMC article. Review.
-
ID1 and CEBPA coordinate epidermal progenitor cell differentiation.Development. 2022 Nov 15;149(22):dev201262. doi: 10.1242/dev.201262. Epub 2022 Nov 16. Development. 2022. PMID: 36330928 Free PMC article.
References
-
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Arden KC. FoxO: linking new signaling pathways. Mol Cell. 2004;14:416–418. - PubMed
-
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
-
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. - PubMed
-
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

