Immune cell inhibition by SLAMF7 is mediated by a mechanism requiring src kinases, CD45, and SHIP-1 that is defective in multiple myeloma cells
- PMID: 25312647
- PMCID: PMC4295394
- DOI: 10.1128/MCB.01107-14
Immune cell inhibition by SLAMF7 is mediated by a mechanism requiring src kinases, CD45, and SHIP-1 that is defective in multiple myeloma cells
Abstract
Signaling lymphocytic activation molecule F7 (SLAMF7) is a receptor present on immune cells, including natural killer (NK) cells. It is also expressed on multiple myeloma (MM) cells. This led to development of an anti-SLAMF7 antibody, elotuzumab, showing efficacy against MM. SLAMF7 mediates activating or inhibitory effects in NK cells, depending on whether cells express or do not express the adaptor EAT-2. Since MM cells lack EAT-2, we elucidated the inhibitory effectors of SLAMF7 in EAT-2-negative NK cells and tested whether these effectors were triggered in MM cells. SLAMF7-mediated inhibition in NK cells lacking EAT-2 was mediated by SH2 domain-containing inositol phosphatase 1 (SHIP-1), which was recruited via tyrosine 261 of SLAMF7. Coupling of SLAMF7 to SHIP-1 required Src kinases, which phosphorylated SLAMF7. Although MM cells lack EAT-2, elotuzumab did not induce inhibitory signals in these cells. This was at least partly due to a lack of CD45, a phosphatase required for Src kinase activation. A defect in SLAMF7 function was also observed in CD45-deficient NK cells. Hence, SLAMF7-triggered inhibition is mediated by a mechanism involving Src kinases, CD45, and SHIP-1 that is defective in MM cells. This defect might explain why elotuzumab eliminates MM cells by an indirect mechanism involving the activation of NK cells.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

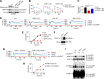



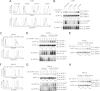
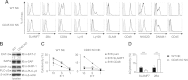
References
-
- Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, Rice AG, van Abbema A, Wong M, Liu G, Zhan F, Dillon M, Chen S, Rhodes S, Fuh F, Tsurushita N, Kumar S, Vexler V, Shaughnessy JD Jr, Barlogie B, van Rhee F, Hussein M, Afar DE, Williams MB. 2008. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res 14:2775–2784. doi:10.1158/1078-0432.CCR-07-4246. - DOI - PMC - PubMed
-
- Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC. 2008. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 112:1329–1337. doi:10.1182/blood-2007-08-107292. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
