Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge
- PMID: 25312762
- PMCID: PMC4587899
- DOI: 10.1016/j.jaci.2014.08.014
Eosinophils contribute to the resolution of lung-allergic responses following repeated allergen challenge
Abstract
Background: Eosinophils accumulate at the site of allergic inflammation and are critical effector cells in allergic diseases. Recent studies have also suggested a role for eosinophils in the resolution of inflammation.
Objective: To determine the role of eosinophils in the resolution phase of the response to repeated allergen challenge.
Methods: Eosinophil-deficient (PHIL) and wild-type (WT) littermates were sensitized and challenged to ovalbumin (OVA) 7 or 11 times. Airway inflammation, airway hyperresponsiveness (AHR) to inhaled methacholine, bronchoalveolar lavage (BAL) cytokine levels, and lung histology were monitored. Intracellular cytokine levels in BAL leukocytes were analyzed by flow cytometry. Groups of OVA-sensitized PHIL mice received bone marrow from WT or IL-10(-/-) donors 30 days before the OVA challenge.
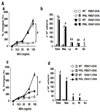
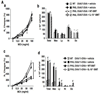
Results: PHIL and WT mice developed similar levels of AHR and numbers of leukocytes and cytokine levels in BAL fluid after OVA sensitization and 7 airway challenges; no eosinophils were detected in the PHIL mice. Unlike WT mice, sensitized PHIL mice maintained AHR, lung inflammation, and increased levels of IL-4, IL-5, and IL-13 in BAL fluid after 11 challenges whereas IL-10 and TGF-β levels were decreased. Restoration of eosinophil numbers after injection of bone marrow from WT but not IL-10-deficient mice restored levels of IL-10 and TGF-β in BAL fluid as well as suppressed AHR and inflammation. Intracellular staining of BAL leukocytes revealed the capacity of eosinophils to produce IL-10.
Conclusions: After repeated allergen challenge, eosinophils appeared not essential for the development of AHR and lung inflammation but contributed to the resolution of AHR and inflammation by producing IL-10.
Keywords: Eosinophils; IL-10; resolution of inflammation.
Copyright © 2014 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures








Comment in
-
A survivor: the eosinophil as a regulator in asthma.J Allergy Clin Immunol. 2015 Feb;135(2):461-2. doi: 10.1016/j.jaci.2014.10.054. Epub 2014 Dec 19. J Allergy Clin Immunol. 2015. PMID: 25533648 No abstract available.
References
-
- Bousquet J, Mantzouranis E, Cruz AA, Ait-Khaled N, Baena-Cagnani, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–938. - PubMed
-
- Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol. 2003;111:227–242. quiz 243. - PubMed
-
- Wills-Karp M, Karp CL. Biomedicine. Eosinophils in asthma: remodeling a tangled tale. Science. 2004;305:1726–1729. - PubMed
-
- Kallinich T, Beier KC, Wahn U, Stock P, Hamelmann E. T-cell co-stimulatory molecules: their role in allergic immune reactions. Eur Respir J. 2007;29:1246–1255. - PubMed
-
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

