Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice
- PMID: 25312824
- PMCID: PMC4316187
- DOI: 10.1002/eji.201444651
Batf3-dependent CD103+ dendritic cells are major producers of IL-12 that drive local Th1 immunity against Leishmania major infection in mice
Abstract
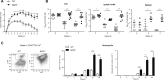
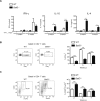
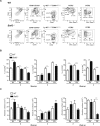
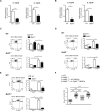
The role of different DC subsets in priming and maintenance of immunity against Leishmania major (L. major) infection is debated. The transcription factor basic leucine zipper transcription factor, ATF-like 3 (Batf3) is essential for the development of mouse CD103(+) DCs and some functions of CD8α(+) DCs. We found that CD103(+) DCs were significantly reduced in the dermis of Batf3-deficient C57BL/6 mice. Batf3(-/-) mice developed exacerbated and unresolved cutaneous pathology following a low dose of intradermal L. major infection in the ear pinnae. Parasite load was increased 1000-fold locally and expanded systemically. Batf3 deficiency did not affect L. major antigen presentation to T cells, which was directly exerted by CD8α(-) conventional DCs (cDCs) in the skin draining LN. However, CD4(+) T-cell differentiation in the LN and skin was skewed to nonprotective Treg- and Th2-cell subtypes. CD103(+) DCs are major IL-12 producers during L. major infection. Local Th1 immunity was severely hindered, correlating with impaired IL-12 production and reduction in CD103(+) DC numbers. Adoptive transfer of WT but not IL-12p40(-/-) Batf3-dependent DCs significantly improved anti-L. major response in infected Batf3(-/-) mice. Our results suggest that IL-12 production by Batf3-dependent CD103(+) DCs is crucial for maintenance of local Th1 immunity against L. major infection.
Keywords: Adaptive immune response ⋅ Batf3 ⋅ Dendritic cells ⋅ IL-12 ⋅ Leishmania major.
© 2014 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

Similar articles
-
Cross-presenting dendritic cells are required for control of Leishmania major infection.Eur J Immunol. 2014 May;44(5):1422-32. doi: 10.1002/eji.201344242. Epub 2014 Mar 25. Eur J Immunol. 2014. PMID: 24643576
-
Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells.J Exp Med. 2010 Apr 12;207(4):823-36. doi: 10.1084/jem.20091627. Epub 2010 Mar 29. J Exp Med. 2010. PMID: 20351058 Free PMC article.
-
Batf3-dependent CD103(+) dendritic cell accumulation is dispensable for mucosal and systemic antifungal host defense.Virulence. 2016 Oct 2;7(7):826-35. doi: 10.1080/21505594.2016.1186324. Epub 2016 May 18. Virulence. 2016. PMID: 27191829 Free PMC article.
-
Requirements for Th1-dependent immunity against infection with Leishmania major.Microbes Infect. 2004 Oct;6(12):1102-9. doi: 10.1016/j.micinf.2004.05.024. Microbes Infect. 2004. PMID: 15380780 Review.
-
Concise review: The heterogenous roles of BATF3 in cancer oncogenesis and dendritic cells and T cells differentiation and function considering the importance of BATF3-dependent dendritic cells.Immunogenetics. 2024 Apr;76(2):75-91. doi: 10.1007/s00251-024-01335-x. Epub 2024 Feb 15. Immunogenetics. 2024. PMID: 38358555 Review.
Cited by
-
The expanding Pandora's toolbox of CD8+T cell: from transcriptional control to metabolic firing.J Transl Med. 2023 Dec 11;21(1):905. doi: 10.1186/s12967-023-04775-3. J Transl Med. 2023. PMID: 38082437 Free PMC article. Review.
-
BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues.PLoS Pathog. 2019 Jun 12;15(6):e1007866. doi: 10.1371/journal.ppat.1007866. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31188899 Free PMC article.
-
Uncovering potential single nucleotide polymorphisms, copy number variations and related signaling pathways in primary Sjogren's syndrome.Bioengineered. 2021 Dec;12(2):9313-9331. doi: 10.1080/21655979.2021.2000245. Bioengineered. 2021. PMID: 34723755 Free PMC article.
-
Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses.Cell Mol Immunol. 2020 Jun;17(6):587-599. doi: 10.1038/s41423-020-0465-0. Epub 2020 May 20. Cell Mol Immunol. 2020. PMID: 32433540 Free PMC article. Review.
-
The Paradox of a Phagosomal Lifestyle: How Innate Host Cell-Leishmania amazonensis Interactions Lead to a Progressive Chronic Disease.Front Immunol. 2021 Sep 7;12:728848. doi: 10.3389/fimmu.2021.728848. eCollection 2021. Front Immunol. 2021. PMID: 34557194 Free PMC article. Review.
References
-
- Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 2000;165:969–977. - PubMed
-
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003;3:984–993. - PubMed
-
- Ritter U, Osterloh A. A new view on cutaneous dendritic cell subsets in experimental leishmaniasis. Med. Microbiol. Immunol. 2007;196:51–59. - PubMed
-
- Kautz-Neu K, Schwonberg K, Fischer MR, Schermann AI, von Stebut E. Dendritic cells in Leishmania major infections: mechanisms of parasite uptake, cell activation and evidence for physiological relevance. Med. Microbiol. Immunol. 2012;201:581–592. - PubMed
-
- León B, López-Bravo M, Ardavín C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

