Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials
- PMID: 25312998
- PMCID: PMC4357842
- DOI: 10.1158/1055-9965.EPI-14-0745
Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials
Abstract
Background: Implementing optimal lung cancer screening programs requires knowledge of the natural history and detectability of lung cancer. This information can be derived from the results of clinical trials with the aid of microsimulation models.
Methods: Data from the Surveillance, Epidemiology, and End Results (SEER) program and individual-level data from the National Lung Screening Trial (NLST) and the Prostate, Lung, Colon, and Ovarian Cancer Screening trial (PLCO) were used to investigate the sensitivity (by histology and stage) of CT and chest radiography (CXR) and the mean preclinical sojourn time (MPST) of lung cancer (by gender, histology, and stage). The MISCAN-Lung model was used to reproduce the lung cancer incidence by method of detection (clinically or screen-detected), gender, histology, and stage in both trials and SEER, by calibrating CT and CXR sensitivity and natural history parameters.
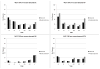
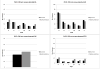
Results: CT sensitivity ranges from 8.83% to 99.35% and CXR sensitivity from 2.51% to 97.31%, depending on histology and stage. CT sensitivity for stage IA is more than 3-fold higher compared with CXR, for all histologies. The total MPST estimates for lung cancer progressing through preclinical stages IA to IV range from 3.09 to 5.32 years for men and 3.35 to 6.01 years for women. The largest difference in total MPST between genders was estimated for adenocarcinoma.
Conclusions: We estimate longer MPSTs for lung cancer compared with previous research, suggesting a greater window of opportunity for lung cancer screening.
Impact: This study provides detailed insights into the natural history of lung cancer and CT screening effectiveness.
©2014 American Association for Cancer Research.
Conflict of interest statement
H.J. de Koning is the principal investigator of the Dutch–Belgian Lung Cancer Screening Trial (Nederlands-Leuvens Longkanker Screenings onderzoek; the NELSON trial). K. ten Haaf and J. van Rosmalen are researchers affiliated with the NELSON trial.
The NELSON-trial received workstations from Siemens Germany for uniform reading across all sites, and funding for a side-study on proteomics by Roche. Roche Diagnostics paid 1,500 Euro to the Erasmus department of public health for a Medical Advisory Board meeting.
Figures

References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: The prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA: The Journal of the American Medical Association. 2011;306:1865–73. - PubMed
-
- de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, et al. Benefits and Harms of Computed Tomography Lung Cancer Screening Strategies: A Comparative Modeling Study for the U.S. Preventive Services Task Force. Ann Intern Med. 2013 doi: 10.7326/M13-2316. published online ahead of print December 31, 2013. - DOI - PMC - PubMed
-
- Fracheboud J, Groenewoud JH, Boer R, Draisma G, de Bruijn AE, Verbeek ALM, et al. Seventy-five years is an appropriate upper age limit for population-based mammography screening. Int J Cancer. 2006;118:2020–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

