Simultaneous targeting of PI3Kδ and a PI3Kδ-dependent MEK1/2-Erk1/2 pathway for therapy in pediatric B-cell acute lymphoblastic leukemia
- PMID: 25313141
- PMCID: PMC4279406
- DOI: 10.18632/oncotarget.2533
Simultaneous targeting of PI3Kδ and a PI3Kδ-dependent MEK1/2-Erk1/2 pathway for therapy in pediatric B-cell acute lymphoblastic leukemia
Abstract
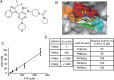
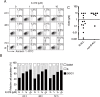
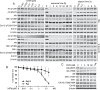
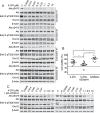
B cell acute lymphoblastic leukemia (B-ALL) is the most common hematological malignancy diagnosed in children, and blockade of the abnormally activated PI3Kδ displayed promising outcomes in B cell acute or chronic leukemias, but the mechanisms are not well understood. Here we report a novel PI3Kδ selective inhibitor X-370, which displays distinct binding mode with p110δ and blocks constitutively active or stimulus-induced PI3Kδ signaling. X-370 significantly inhibited survival of human B cell leukemia cells in vitro, with associated induction of G1 phase arrest and apoptosis. X-370 abrogated both Akt and Erk1/2 signaling via blockade of PDK1 binding to and/or phosphorylation of MEK1/2. Forced expression of a constitutively active MEK1 attenuated the antiproliferative activity of X-370. X-370 preferentially inhibited the survival of primary pediatric B-ALL cells displaying PI3Kδ-dependent Erk1/2 phosphorylation, while combined inhibition of PI3Kδ and MEK1/2 displayed enhanced activity. We conclude that PI3Kδ inhibition led to abrogation of both Akt and Erk1/2 signaling via a novel PI3K-PDK1/MEK1/2-Erk1/2 signaling cascade, which contributed to its efficacy against B-ALL. These findings support the rationale for clinical testing of PI3Kδ inhibitors in pediatric B-ALL and provide insights needed to optimize the therapeutic strategy.
Figures


Similar articles
-
Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines.Int J Cancer. 2009 Nov 15;125(10):2332-41. doi: 10.1002/ijc.24604. Int J Cancer. 2009. PMID: 19637312
-
Primary cross-resistance to BRAFV600E-, MEK1/2- and PI3K/mTOR-specific inhibitors in BRAF-mutant melanoma cells counteracted by dual pathway blockade.Oncotarget. 2016 Jan 26;7(4):3947-65. doi: 10.18632/oncotarget.6600. Oncotarget. 2016. PMID: 26678033 Free PMC article.
-
Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways.Placenta. 2009 Dec;30(12):1045-51. doi: 10.1016/j.placenta.2009.10.007. Epub 2009 Nov 5. Placenta. 2009. PMID: 19892399 Free PMC article.
-
Targeting ERK1/2 protein-serine/threonine kinases in human cancers.Pharmacol Res. 2019 Apr;142:151-168. doi: 10.1016/j.phrs.2019.01.039. Epub 2019 Feb 20. Pharmacol Res. 2019. PMID: 30794926 Review.
-
MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road.Nat Rev Cancer. 2015 Oct;15(10):577-92. doi: 10.1038/nrc4000. Nat Rev Cancer. 2015. PMID: 26399658 Review.
Cited by
-
Integration of Receptor Tyrosine Kinases Determines Sensitivity to PI3Kα-selective Inhibitors in Breast Cancer.Theranostics. 2017 Feb 23;7(4):974-986. doi: 10.7150/thno.17830. eCollection 2017. Theranostics. 2017. PMID: 28382169 Free PMC article.
-
PI3K isoform-selective inhibitors: next-generation targeted cancer therapies.Acta Pharmacol Sin. 2015 Oct;36(10):1170-6. doi: 10.1038/aps.2015.71. Epub 2015 Sep 14. Acta Pharmacol Sin. 2015. PMID: 26364801 Free PMC article. Review.
-
A Review of Phosphocreatine 3 Kinase δ Subtype (PI3Kδ) and Its Inhibitors in Malignancy.Med Sci Monit. 2021 Oct 9;27:e932772. doi: 10.12659/MSM.932772. Med Sci Monit. 2021. PMID: 34625526 Free PMC article. Review.
-
Glucose Catabolism in Liver Tumors Induced by c-MYC Can Be Sustained by Various PKM1/PKM2 Ratios and Pyruvate Kinase Activities.Cancer Res. 2017 Aug 15;77(16):4355-4364. doi: 10.1158/0008-5472.CAN-17-0498. Epub 2017 Jun 19. Cancer Res. 2017. PMID: 28630053 Free PMC article.
-
PI3K pan-inhibition impairs more efficiently proliferation and survival of T-cell acute lymphoblastic leukemia cell lines when compared to isoform-selective PI3K inhibitors.Oncotarget. 2015 Apr 30;6(12):10399-414. doi: 10.18632/oncotarget.3295. Oncotarget. 2015. PMID: 25871383 Free PMC article.
References
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043. - PubMed
-
- Kiraly JF, 3rd, Wheby MS. Bone marrow necrosis. The American journal of medicine. 1976;60(3):361–368. - PubMed
-
- Greaves MF, Chan LC, Furley AJ, Watt SM, Molgaard HV. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986;67(1):1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

