Peripheral Blood-Derived Mesenchymal Stromal Cells Promote Angiogenesis via Paracrine Stimulation of Vascular Endothelial Growth Factor Secretion in the Equine Model
- PMID: 25313202
- PMCID: PMC4250216
- DOI: 10.5966/sctm.2014-0138
Peripheral Blood-Derived Mesenchymal Stromal Cells Promote Angiogenesis via Paracrine Stimulation of Vascular Endothelial Growth Factor Secretion in the Equine Model
Abstract
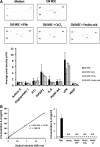
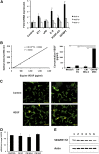
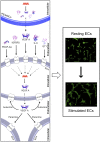
Mesenchymal stromal cells (MSCs) have received much attention as a potential treatment of ischemic diseases, including ischemic tissue injury and cardiac failure. The beneficial effects of MSCs are thought to be mediated by their ability to provide proangiogenic factors, creating a favorable microenvironment that results in neovascularization and tissue regeneration. To study this in more detail and to explore the potential of the horse as a valuable translational model, the objectives of the present study were to examine the presence of angiogenic stimulating factors in the conditioned medium (CM) of peripheral blood-derived equine mesenchymal stromal cells (PB-MSCs) and to study their in vitro effect on angiogenesis-related endothelial cell (EC) behavior, including proliferation and vessel formation. Our salient findings were that CM from PB-MSCs contained significant levels of several proangiogenic factors. Furthermore, we found that CM could induce angiogenesis in equine vascular ECs and confirmed that endothelin-1, insulin growth factor binding protein 2, interleukin-8, and platelet-derived growth factor-AA, but not urokinase-type plasminogen activator, were responsible for this enhanced EC network formation by increasing the expression level of vascular endothelial growth factor-A, an important angiogenesis stimulator.
Keywords: Angiogenesis; Mesenchymal stromal cells; Secretome; Vascular endothelial growth factor.
©AlphaMed Press.
Figures



Similar articles
-
Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro.Stem Cells Dev. 2013 Feb 15;22(4):643-53. doi: 10.1089/scd.2012.0273. Epub 2012 Oct 19. Stem Cells Dev. 2013. PMID: 22947186 Free PMC article. Clinical Trial.
-
An In Vitro Co-Culture Model of Bone Marrow Mesenchymal Stromal Cells and Peripheral Blood Mononuclear Cells Promotes the Differentiation of Myeloid Angiogenic Cells and Pericyte-Like Cells.Stem Cells Dev. 2021 Mar;30(6):309-324. doi: 10.1089/scd.2019.0171. Epub 2021 Mar 1. Stem Cells Dev. 2021. PMID: 33499756
-
Platelet-rich plasma improves the therapeutic efficacy of mesenchymal stem cells by enhancing their secretion of angiogenic factors in a combined radiation and wound injury model.Exp Dermatol. 2020 Feb;29(2):158-167. doi: 10.1111/exd.14042. Epub 2019 Nov 18. Exp Dermatol. 2020. PMID: 31560791
-
Angiogenic Effects and Crosstalk of Adipose-Derived Mesenchymal Stem/Stromal Cells and Their Extracellular Vesicles with Endothelial Cells.Int J Mol Sci. 2021 Oct 8;22(19):10890. doi: 10.3390/ijms221910890. Int J Mol Sci. 2021. PMID: 34639228 Free PMC article. Review.
-
Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine.Int J Mol Sci. 2021 Jan 14;22(2):763. doi: 10.3390/ijms22020763. Int J Mol Sci. 2021. PMID: 33466583 Free PMC article. Review.
Cited by
-
Microencapsulated equine mesenchymal stromal cells promote cutaneous wound healing in vitro.Stem Cell Res Ther. 2015 Apr 11;6(1):66. doi: 10.1186/s13287-015-0037-x. Stem Cell Res Ther. 2015. PMID: 25889766 Free PMC article.
-
Vascularization in tissue engineering: fundamentals and state-of-art.Prog Biomed Eng (Bristol). 2020 Jan;2(1):012002. doi: 10.1088/2516-1091/ab5637. Epub 2020 Jan 9. Prog Biomed Eng (Bristol). 2020. PMID: 34308105 Free PMC article.
-
Stem cells mobilization by cardiopulmonary bypass after coronary artery bypass grafting.Postepy Kardiol Interwencyjnej. 2022 Dec;18(4):450-458. doi: 10.5114/aic.2022.122874. Epub 2022 Dec 17. Postepy Kardiol Interwencyjnej. 2022. PMID: 36967861 Free PMC article.
-
Curcumin preconditioning enhances the efficacy of adipose-derived mesenchymal stem cells to accelerate healing of burn wounds.Burns Trauma. 2021 Sep 11;9:tkab021. doi: 10.1093/burnst/tkab021. eCollection 2021. Burns Trauma. 2021. PMID: 34514007 Free PMC article.
-
Microvesicle-mediated Wnt/β-Catenin Signaling Promotes Interspecies Mammary Stem/Progenitor Cell Growth.J Biol Chem. 2016 Nov 18;291(47):24390-24405. doi: 10.1074/jbc.M116.726117. Epub 2016 Oct 12. J Biol Chem. 2016. PMID: 27733685 Free PMC article.
References
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. - PubMed
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Sunkomat JNE, Gaballa MA. Stem cell therapy in ischemic heart disease. Cardiovasc Drug Rev. 2003;21:327–342. - PubMed
-
- Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

