Impact on resistance of the use of therapeutically equivalent generics: the case of ciprofloxacin
- PMID: 25313208
- PMCID: PMC4291395
- DOI: 10.1128/AAC.03633-14
Impact on resistance of the use of therapeutically equivalent generics: the case of ciprofloxacin
Abstract
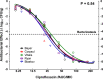
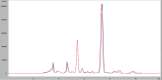
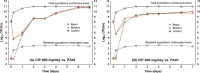
Therapeutic nonequivalence of generic antibiotics may lead to treatment failure and enrichment of resistance. However, there has been no demonstration that an equivalent generic displays the same resistance selection profile as the innovator drug. We aimed to test this hypothesis with five generic versions of ciprofloxacin by assessing their pharmaceutical equivalence with microbiological assays and their efficacy against Pseudomonas aeruginosa PAO1 in the neutropenic murine thigh infection model. One equivalent generic was selected for analysis by high-pressure liquid chromatography-tandem mass spectrometry (LC-MS/MS), to confirm chemical identity, and resistance selection experiments in a hollow-fiber (HF) system simulating two clinical dosing regimens. Total and resistant populations were measured, and the MICs of the resistant cells with and without an efflux pump inhibitor were determined. LC-MS/MS found no differences between products, and the innovator and the generic selected resistance with the same magnitude and mechanism after 7 days of treatment in the HF system, supporting the fact that a generic with demonstrated equivalence in vivo is also equivalent regarding resistance selection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Impact on Bacterial Resistance of Therapeutically Nonequivalent Generics: The Case of Piperacillin-Tazobactam.PLoS One. 2016 May 18;11(5):e0155806. doi: 10.1371/journal.pone.0155806. eCollection 2016. PLoS One. 2016. PMID: 27191163 Free PMC article.
-
Even apparently insignificant chemical deviations among bioequivalent generic antibiotics can lead to therapeutic nonequivalence: the case of meropenem.Antimicrob Agents Chemother. 2014;58(2):1005-18. doi: 10.1128/AAC.00350-13. Epub 2013 Nov 25. Antimicrob Agents Chemother. 2014. PMID: 24277034 Free PMC article.
-
Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator.Antimicrob Agents Chemother. 2010 Aug;54(8):3271-9. doi: 10.1128/AAC.01044-09. Epub 2010 Jun 14. Antimicrob Agents Chemother. 2010. PMID: 20547818 Free PMC article.
-
Inhaled ciprofloxacin for chronic airways infections caused by Pseudomonas aeruginosa.Expert Rev Anti Infect Ther. 2012 Dec;10(12):1439-46. doi: 10.1586/eri.12.136. Expert Rev Anti Infect Ther. 2012. PMID: 23253321 Review.
-
Review of resistance of ocular isolates of Pseudomonas aeruginosa and staphylococci from keratitis to ciprofloxacin, gentamicin and cephalosporins.Clin Exp Optom. 2011 Mar;94(2):161-8. doi: 10.1111/j.1444-0938.2010.00536.x. Epub 2010 Nov 17. Clin Exp Optom. 2011. PMID: 21083760 Review.
Cited by
-
Interplay of the Quality of Ciprofloxacin and Antibiotic Resistance in Developing Countries.Front Pharmacol. 2017 Aug 21;8:546. doi: 10.3389/fphar.2017.00546. eCollection 2017. Front Pharmacol. 2017. PMID: 28871228 Free PMC article.
-
Demonstration of Therapeutic Equivalence of Fluconazole Generic Products in the Neutropenic Mouse Model of Disseminated Candidiasis.PLoS One. 2015 Nov 4;10(11):e0141872. doi: 10.1371/journal.pone.0141872. eCollection 2015. PLoS One. 2015. PMID: 26536105 Free PMC article.
-
Impact on Bacterial Resistance of Therapeutically Nonequivalent Generics: The Case of Piperacillin-Tazobactam.PLoS One. 2016 May 18;11(5):e0155806. doi: 10.1371/journal.pone.0155806. eCollection 2016. PLoS One. 2016. PMID: 27191163 Free PMC article.
-
In Vitro Antimicrobial Activity of Various Cefoperazone/Sulbactam Products.Antibiotics (Basel). 2020 Feb 12;9(2):77. doi: 10.3390/antibiotics9020077. Antibiotics (Basel). 2020. PMID: 32059590 Free PMC article.
-
Nontherapeutic equivalence of a generic product of imipenem-cilastatin is caused more by chemical instability of the active pharmaceutical ingredient (imipenem) than by its substandard amount of cilastatin.PLoS One. 2019 Feb 6;14(2):e0211096. doi: 10.1371/journal.pone.0211096. eCollection 2019. PLoS One. 2019. PMID: 30726248 Free PMC article.
References
-
- Gonzalez JM, Agudelo M, Leiva LM, Rodriguez CA, Vesga O. 2012. Standardization of a murine model of Candida albicans infection to study therapeutic equivalence (TE) of fluconazole (FCZ) generics, abstr A-1945. Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

