Mass balance, metabolite profile, and in vitro-in vivo comparison of clearance pathways of deleobuvir, a hepatitis C virus polymerase inhibitor
- PMID: 25313217
- PMCID: PMC4291358
- DOI: 10.1128/AAC.03861-14
Mass balance, metabolite profile, and in vitro-in vivo comparison of clearance pathways of deleobuvir, a hepatitis C virus polymerase inhibitor
Abstract
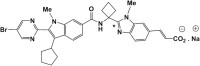
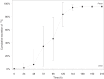
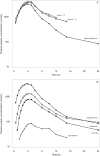
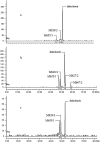
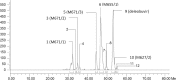
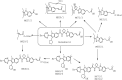
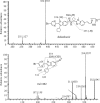
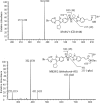
The pharmacokinetics, mass balance, and metabolism of deleobuvir, a hepatitis C virus (HCV) polymerase inhibitor, were assessed in healthy subjects following a single oral dose of 800 mg of [(14)C]deleobuvir (100 μCi). The overall recovery of radioactivity was 95.2%, with 95.1% recovered from feces. Deleobuvir had moderate to high clearance, and the half-life of deleobuvir and radioactivity in plasma were ∼ 3 h, indicating that there were no metabolites with half-lives significantly longer than that of the parent. The most frequently reported adverse events (in 6 of 12 subjects) were gastrointestinal disorders. Two major metabolites of deleobuvir were identified in plasma: an acyl glucuronide and an alkene reduction metabolite formed in the gastrointestinal (GI) tract by gut bacteria (CD 6168), representing ∼ 20% and 15% of the total drug-related material, respectively. Deleobuvir and CD 6168 were the main components in the fecal samples, each representing ∼ 30 to 35% of the dose. The majority of the remaining radioactivity found in the fecal samples (∼ 21% of the dose) was accounted for by three metabolites in which deleobuvir underwent both alkene reduction and monohydroxylation. In fresh human hepatocytes that form biliary canaliculi in sandwich cultures, the biliary excretion for these excretory metabolites was markedly higher than that for deleobuvir and CD 6168, implying that rapid biliary elimination upon hepatic formation may underlie the absence of these metabolites in circulation. The low in vitro clearance was not predictive of the observed in vivo clearance, likely because major deleobuvir biotransformation occurred by non-CYP450-mediated enzymes that are not well represented in hepatocyte-based in vitro models.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Defining the Role of Gut Bacteria in the Metabolism of Deleobuvir: In Vitro and In Vivo Studies.Drug Metab Dispos. 2015 Oct;43(10):1612-8. doi: 10.1124/dmd.115.064477. Epub 2015 Jun 11. Drug Metab Dispos. 2015. PMID: 26068924
-
Contribution of Major Metabolites toward Complex Drug-Drug Interactions of Deleobuvir: In Vitro Predictions and In Vivo Outcomes.Drug Metab Dispos. 2016 Mar;44(3):466-75. doi: 10.1124/dmd.115.066985. Epub 2015 Dec 18. Drug Metab Dispos. 2016. PMID: 26684498
-
Antiviral effect, safety, and pharmacokinetics of five-day oral administration of Deleobuvir (BI 207127), an investigational hepatitis C virus RNA polymerase inhibitor, in patients with chronic hepatitis C.Antimicrob Agents Chemother. 2013 Oct;57(10):4727-35. doi: 10.1128/AAC.00565-13. Epub 2013 Jul 15. Antimicrob Agents Chemother. 2013. PMID: 23856779 Free PMC article. Clinical Trial.
-
HCVerso3: An Open-Label, Phase IIb Study of Faldaprevir and Deleobuvir with Ribavirin in Hepatitis C Virus Genotype-1b-Infected Patients with Cirrhosis and Moderate Hepatic Impairment.PLoS One. 2016 Dec 28;11(12):e0168544. doi: 10.1371/journal.pone.0168544. eCollection 2016. PLoS One. 2016. PMID: 28030579 Free PMC article. Clinical Trial.
-
Absorption, Distribution, Metabolism, and Excretion of the Oral Prostaglandin D2 Receptor 2 Antagonist Fevipiprant (QAW039) in Healthy Volunteers and In Vitro.Drug Metab Dispos. 2017 Jul;45(7):817-825. doi: 10.1124/dmd.117.075358. Epub 2017 Apr 25. Drug Metab Dispos. 2017. PMID: 28442499 Clinical Trial.
Cited by
-
Current therapy for chronic hepatitis C: The role of direct-acting antivirals.Antiviral Res. 2017 Jun;142:83-122. doi: 10.1016/j.antiviral.2017.02.014. Epub 2017 Feb 24. Antiviral Res. 2017. PMID: 28238877 Free PMC article. Review.
References
-
- World Health Organization. 2014. Hepatitis C fact sheet. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs164/en/.
-
- Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. 1999. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 341:556–562. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958–965. doi:10.1016/S0140-6736(01)06102-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

