β-catenin links von Hippel-Lindau to aurora kinase A and loss of primary cilia in renal cell carcinoma
- PMID: 25313256
- PMCID: PMC4341469
- DOI: 10.1681/ASN.2013090984
β-catenin links von Hippel-Lindau to aurora kinase A and loss of primary cilia in renal cell carcinoma
Abstract
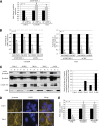
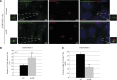
von Hippel-Lindau (VHL) gene mutations are associated with clear cell renal cell carcinoma (ccRCC). A hallmark of ccRCC is loss of the primary cilium. Loss of this key organelle in ccRCC is caused by loss of VHL and associated with increased Aurora kinase A (AURKA) and histone deacetylase 6 (HDAC6) activities, which drive disassembly of the primary cilium. However, the underlying mechanism by which VHL loss increases AURKA levels has not been clearly elucidated, although it has been suggested that hypoxia-inducible factor-1α (HIF-1α) mediates increased AURKA expression in VHL-null cells. By contrast, we found that elevated AURKA expression is not increased by HIF-1α, suggesting an alternate mechanism for AURKA dysregulation in VHL-null cells. We report here that AURKA expression is driven by β-catenin transcription in VHL-null cells. In a panel of RCC cell lines, we observed nuclear accumulation of β-catenin and increased AURKA signaling to HDAC6. Moreover, HIF-1α inhibited AURKA expression by inhibiting β-catenin transcription. VHL knockdown activated β-catenin and elevated AURKA expression, decreased primary cilia formation, and caused significant shortening of cilia length in cells that did form cilia. The β-catenin responsive transcription inhibitor iCRT14 reduced AURKA levels and rescued ciliary defects, inducing a significant increase in primary cilia formation in VHL-deficient cells. These data define a role for β-catenin in regulating AURKA and formation of primary cilia in the setting of VHL deficiency, opening new avenues for treatment with β-catenin inhibitors to rescue ciliogenesis in ccRCC.
Keywords: cell signaling; kidney cancer; renal cell biology.
Copyright © 2015 by the American Society of Nephrology.
Figures





Similar articles
-
Therapeutically actionable signaling node to rescue AURKA driven loss of primary cilia in VHL-deficient cells.Sci Rep. 2021 May 17;11(1):10461. doi: 10.1038/s41598-021-89933-7. Sci Rep. 2021. PMID: 34002003 Free PMC article.
-
Bexarotene - a novel modulator of AURKA and the primary cilium in VHL-deficient cells.J Cell Sci. 2018 Dec 14;131(24):jcs219923. doi: 10.1242/jcs.219923. J Cell Sci. 2018. PMID: 30518623 Free PMC article.
-
Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition.BMC Cancer. 2012 Jul 17;12:293. doi: 10.1186/1471-2407-12-293. BMC Cancer. 2012. PMID: 22804960 Free PMC article.
-
Von hippel-lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development.Cancer Res. 2007 May 15;67(10):4537-40. doi: 10.1158/0008-5472.CAN-07-0391. Cancer Res. 2007. PMID: 17510376 Review.
-
von-Hippel Lindau and Hypoxia-Inducible Factor at the Center of Renal Cell Carcinoma Biology.Hematol Oncol Clin North Am. 2023 Oct;37(5):809-825. doi: 10.1016/j.hoc.2023.04.011. Epub 2023 Jun 1. Hematol Oncol Clin North Am. 2023. PMID: 37270382 Free PMC article. Review.
Cited by
-
Primary cilia: a novel research approach to overcome anticancer drug resistance.Front Mol Biosci. 2023 Oct 2;10:1270639. doi: 10.3389/fmolb.2023.1270639. eCollection 2023. Front Mol Biosci. 2023. PMID: 37900915 Free PMC article. Review.
-
Recent advances in the understanding of cilia mechanisms and their applications as therapeutic targets.Front Mol Biosci. 2023 Sep 14;10:1232188. doi: 10.3389/fmolb.2023.1232188. eCollection 2023. Front Mol Biosci. 2023. PMID: 37780208 Free PMC article. Review.
-
Olfactory Loss and Dysfunction in Ciliopathies: Molecular Mechanisms and Potential Therapies.Curr Med Chem. 2019;26(17):3103-3119. doi: 10.2174/0929867325666180105102447. Curr Med Chem. 2019. PMID: 29303074 Free PMC article. Review.
-
Cilia and Cancer: From Molecular Genetics to Therapeutic Strategies.Genes (Basel). 2023 Jul 11;14(7):1428. doi: 10.3390/genes14071428. Genes (Basel). 2023. PMID: 37510333 Free PMC article. Review.
-
The primary cilium: Its role as a tumor suppressor organelle.Biochem Pharmacol. 2020 May;175:113906. doi: 10.1016/j.bcp.2020.113906. Epub 2020 Mar 10. Biochem Pharmacol. 2020. PMID: 32169416 Free PMC article. Review.
References
-
- Bausch B, Jilg C, Gläsker S, Vortmeyer A, Lützen N, Anton A, Eng C, Neumann HP: Renal cancer in von Hippel-Lindau disease and related syndromes. Nat Rev Nephrol 9: 529–538, 2013 - PubMed
-
- Valladares Ayerbes M, Aparicio Gallego G, Díaz Prado S, Jiménez Fonseca P, García Campelo R, Antón Aparicio LM: Origin of renal cell carcinomas. Clin Transl Oncol 10: 697–712, 2008 - PubMed
-
- Stebbins CE, Kaelin WG, Jr, Pavletich NP: Structure of the VHL-ElonginC-ElonginB complex: Implications for VHL tumor suppressor function. Science 284: 455–461, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

