Pentachlorophenol degradation by Janibacter sp., a new actinobacterium isolated from saline sediment of arid land
- PMID: 25313357
- PMCID: PMC4182692
- DOI: 10.1155/2014/296472
Pentachlorophenol degradation by Janibacter sp., a new actinobacterium isolated from saline sediment of arid land
Abstract
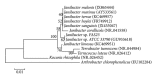
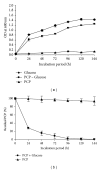
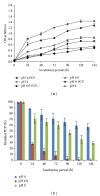
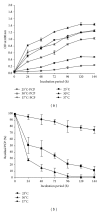
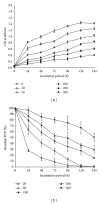
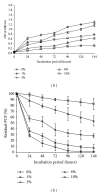
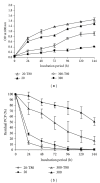
Many pentachlorophenol- (PCP-) contaminated environments are characterized by low or elevated temperatures, acidic or alkaline pH, and high salt concentrations. PCP-degrading microorganisms, adapted to grow and prosper in these environments, play an important role in the biological treatment of polluted extreme habitats. A PCP-degrading bacterium was isolated and characterized from arid and saline soil in southern Tunisia and was enriched in mineral salts medium supplemented with PCP as source of carbon and energy. Based on 16S rRNA coding gene sequence analysis, the strain FAS23 was identified as Janibacter sp. As revealed by high performance liquid chromatography (HPLC) analysis, FAS23 strain was found to be efficient for PCP removal in the presence of 1% of glucose. The conditions of growth and PCP removal by FAS23 strain were found to be optimal in neutral pH and at a temperature of 30 °C. Moreover, this strain was found to be halotolerant at a range of 1-10% of NaCl and able to degrade PCP at a concentration up to 300 mg/L, while the addition of nonionic surfactant (Tween 80) enhanced the PCP removal capacity.
Figures
References
-
- Vallecillo A, Garcia-Encina PA, Peña M. Anaerobic biodegradability and toxicity of chlorophenols. Water Science and Technology. 1999;40(8):161–168.
-
- Kao CM, Chai CT, Liu JK, Yeh TY, Chen KF, Chen SC. Evaluation of natural and enhanced PCP biodegradation at a former pesticide manufacturing plant. Water Research. 2004;38(3):663–672. - PubMed
-
- Shen D-S, Liu X-W, Feng H-J. Effect of easily degradable substrate on anaerobic degradation of pentachlorophenol in an upflow anaerobic sludge blanket (UASB) reactor. Journal of Hazardous Materials. 2005;119(1–3):239–243. - PubMed
-
- Sai K, Kang KS, Hirose A, Hasegawa R, Trosko JE, Inoue T. Inhibition of apoptosis by pentachlorophenol in v-myc-transfected rat liver epithelial cells: relation to down-regulation of gap junctional intercellular communication. Cancer Letters. 2001;173(2):163–174. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

