Coating of mesh grafts for prolapse and urinary incontinence repair with autologous plasma: exploration stage of a surgical innovation
- PMID: 25313358
- PMCID: PMC4182302
- DOI: 10.1155/2014/296498
Coating of mesh grafts for prolapse and urinary incontinence repair with autologous plasma: exploration stage of a surgical innovation
Abstract
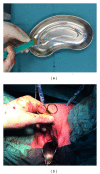
Purpose: Optimized biocompatibility is a major requirement for alloplastic materials currently applied for stress urinary incontinence (SUI) and pelvic organ prolapse (POP) repair. In the preliminary studies the mesh modification by coating with autologous plasma resulted in the increased adherence score in vitro and improved biocompatibility in an animal model. The first use of plasma coated meshes in human is presented.
Materials and methods: Between 04/2013 and 05/2014, 20 patients with the indication for SUI and POP repair were selected in a single institution. The applied meshes were modified by autologous plasma coating prior to implantation. A retrospective chart review for peri- and early postoperative complications was performed. Functional outcome and QoL were evaluated pre- and postoperatively.
Results: The functional outcome and QoL improved significantly in all groups. Two reoperations (Grade IIIB) with the release of TVT-mesh in anesthesia due to the obstruction were needed. No other severe complications were registered.
Conclusion: For the first time we applied a mesh modification in a human setting according to IDEAL criteria of surgical innovations. The procedure of mesh coating with autologous plasma is safe and a prospective randomized trial proving a positive effect of plasma coating on the biocompatibility and morbidity outcome with long-term registry is planned.
Figures
References
-
- UPDATE on Serious Complications Associated with Transvaginal Placement of Surgical Mesh for Pelvic Organ Prolapse: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm. - PubMed
-
- Haylen BT, Freeman RM, Swift SE. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourology and Urodynamics. 2011;30(1):2–12. - PubMed
-
- Amid PK, Lichtenstein IL. Current situation of the Lichtenstein open tension-free hernioplasty. Chirurg. 1997;68(10):959–964. - PubMed
-
- Barski D, Otto T, Gerullis H. Systematic review and classification of complications after anterior, posterior, apical, and total vaginal mesh implantation for prolapse repair. Surgical Technology International. 2014;24 - PubMed
-
- Maher C, Feiner B, Baessler K, Schmid C. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews. 2013;(4)CD004014 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

