Quorum sensing regulates the osmotic stress response in Vibrio harveyi
- PMID: 25313392
- PMCID: PMC4288691
- DOI: 10.1128/JB.02246-14
Quorum sensing regulates the osmotic stress response in Vibrio harveyi
Abstract
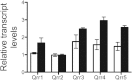
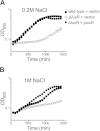
Bacteria use a chemical communication process called quorum sensing to monitor cell density and to alter behavior in response to fluctuations in population numbers. Previous studies with Vibrio harveyi have shown that LuxR, the master quorum-sensing regulator, activates and represses >600 genes. These include six genes that encode homologs of the Escherichia coli Bet and ProU systems for synthesis and transport, respectively, of glycine betaine, an osmoprotectant used during osmotic stress. Here we show that LuxR activates expression of the glycine betaine operon betIBA-proXWV, which enhances growth recovery under osmotic stress conditions. BetI, an autorepressor of the V. harveyi betIBA-proXWV operon, activates the expression of genes encoding regulatory small RNAs that control quorum-sensing transitions. Connecting quorum-sensing and glycine betaine pathways presumably enables V. harveyi to tune its execution of collective behaviors to its tolerance to stress.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Integration host factor and LuxR synergistically bind DNA to coactivate quorum-sensing genes in Vibrio harveyi.Mol Microbiol. 2016 Sep;101(5):823-40. doi: 10.1111/mmi.13425. Epub 2016 Jun 16. Mol Microbiol. 2016. PMID: 27191515
-
Individual and combined roles of the master regulators AphA and LuxR in control of the Vibrio harveyi quorum-sensing regulon.J Bacteriol. 2013 Feb;195(3):436-43. doi: 10.1128/JB.01998-12. Epub 2012 Nov 30. J Bacteriol. 2013. PMID: 23204455 Free PMC article.
-
Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi.Genes Dev. 2007 Jan 15;21(2):221-33. doi: 10.1101/gad.1502407. Genes Dev. 2007. PMID: 17234887 Free PMC article.
-
The osmotic stress response operon betIBA is under the functional regulation of BetI and the quorum-sensing regulator AnoR in Acinetobacter nosocomialis.J Microbiol. 2020 Jun;58(6):519-529. doi: 10.1007/s12275-020-0186-1. Epub 2020 May 27. J Microbiol. 2020. PMID: 32462489 Review.
-
Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios.J Bacteriol. 2017 Sep 5;199(19):e00105-17. doi: 10.1128/JB.00105-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28484045 Free PMC article. Review.
Cited by
-
Autoinducer-2 Quorum Sensing Influences Viability of Escherichia coli O157:H7 under Osmotic and In Vitro Gastrointestinal Stress Conditions.Front Microbiol. 2017 Jun 13;8:1077. doi: 10.3389/fmicb.2017.01077. eCollection 2017. Front Microbiol. 2017. PMID: 28659895 Free PMC article.
-
Exploiting the Feedstock Flexibility of the Emergent Synthetic Biology Chassis Vibrio natriegens for Engineered Natural Product Production.Mar Drugs. 2019 Nov 30;17(12):679. doi: 10.3390/md17120679. Mar Drugs. 2019. PMID: 31801279 Free PMC article.
-
CosR Is a Global Regulator of the Osmotic Stress Response with Widespread Distribution among Bacteria.Appl Environ Microbiol. 2020 May 5;86(10):e00120-20. doi: 10.1128/AEM.00120-20. Print 2020 May 5. Appl Environ Microbiol. 2020. PMID: 32169942 Free PMC article.
-
Role of Non-coding Regulatory RNA in the Virulence of Human Pathogenic Vibrios.Front Microbiol. 2017 Jan 11;7:2160. doi: 10.3389/fmicb.2016.02160. eCollection 2016. Front Microbiol. 2017. PMID: 28123382 Free PMC article. Review.
-
Stressed out: Bacterial response to high salinity using compatible solute biosynthesis and uptake systems, lessons from Vibrionaceae.Comput Struct Biotechnol J. 2021 Feb 1;19:1014-1027. doi: 10.1016/j.csbj.2021.01.030. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33613867 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

