Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation
- PMID: 25313408
- PMCID: PMC4195830
- DOI: 10.1083/jcb.201406140
Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation
Abstract
Airway multiciliated epithelial cells play crucial roles in the mucosal defense system, but their differentiation process remains poorly understood. Mice lacking the basal body component Chibby (Cby) exhibit impaired mucociliary transport caused by defective ciliogenesis, resulting in chronic airway infection. In this paper, using primary cultures of mouse tracheal epithelial cells, we show that Cby facilitates basal body docking to the apical cell membrane through proper formation of ciliary vesicles at the distal appendage during the early stages of ciliogenesis. Cby is recruited to the distal appendages of centrioles via physical interaction with the distal appendage protein CEP164. Cby then associates with the membrane trafficking machinery component Rabin8, a guanine nucleotide exchange factor for the small guanosine triphosphatase Rab8, to promote recruitment of Rab8 and efficient assembly of ciliary vesicles. Thus, our study identifies Cby as a key regulator of ciliary vesicle formation and basal body docking during the differentiation of airway ciliated cells.
© 2014 Burke et al.
Figures
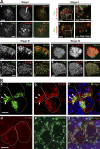
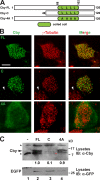
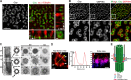
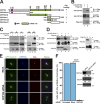
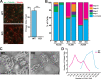
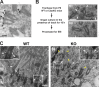
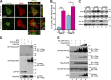

References
-
- Boisvieux-Ulrich, E., Laine M.C., and Sandoz D.. 1989. In vitro effects of taxol on ciliogenesis in quail oviduct. J. Cell Sci. 92:9–20 - PubMed
-
- Chaki, M., Airik R., Ghosh A.K., Giles R.H., Chen R., Slaats G.G., Wang H., Hurd T.W., Zhou W., Cluckey A., et al. . 2012. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 150:533–548 10.1016/j.cell.2012.06.028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

