SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking
- PMID: 25313507
- PMCID: PMC4350523
- DOI: 10.1038/tp.2014.90
SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking
Abstract
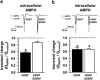
Emerging evidence associates dysfunction in the dopamine (DA) transporter (DAT) with the pathophysiology of autism spectrum disorder (ASD). The human DAT (hDAT; SLC6A3) rare variant with an Ala to Val substitution at amino acid 559 (hDAT A559V) was previously reported in individuals with bipolar disorder or attention-deficit hyperactivity disorder (ADHD). We have demonstrated that this variant is hyper-phosphorylated at the amino (N)-terminal serine (Ser) residues and promotes an anomalous DA efflux phenotype. Here, we report the novel identification of hDAT A559V in two unrelated ASD subjects and provide the first mechanistic description of its impaired trafficking phenotype. DAT surface expression is dynamically regulated by DAT substrates including the psychostimulant amphetamine (AMPH), which causes hDAT trafficking away from the plasma membrane. The integrity of DAT trafficking directly impacts DA transport capacity and therefore dopaminergic neurotransmission. Here, we show that hDAT A559V is resistant to AMPH-induced cell surface redistribution. This unique trafficking phenotype is conferred by altered protein kinase C β (PKCβ) activity. Cells expressing hDAT A559V exhibit constitutively elevated PKCβ activity, inhibition of which restores the AMPH-induced hDAT A559V membrane redistribution. Mechanistically, we link the inability of hDAT A559V to traffic in response to AMPH to the phosphorylation of the five most distal DAT N-terminal Ser. Mutation of these N-terminal Ser to Ala restores AMPH-induced trafficking. Furthermore, hDAT A559V has a diminished ability to transport AMPH, and therefore lacks AMPH-induced DA efflux. Pharmacological inhibition of PKCβ or Ser to Ala substitution in the hDAT A559V background restores AMPH-induced DA efflux while promoting intracellular AMPH accumulation. Although hDAT A559V is a rare variant, it has been found in multiple probands with neuropsychiatric disorders associated with imbalances in DA neurotransmission, including ADHD, bipolar disorder, and now ASD. These findings provide valuable insight into a new cellular phenotype (altered hDAT trafficking) supporting dysregulated DA function in these disorders. They also provide a novel potential target (PKCβ) for therapeutic interventions in individuals with ASD.
Figures




References
-
- Devlin B, Scherer SW. Genetic architecture in autism spectrum disorder. Curr Opin Genet Dev. 2012;22:229–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

