L-Endoglin overexpression increases renal fibrosis after unilateral ureteral obstruction
- PMID: 25313562
- PMCID: PMC4196986
- DOI: 10.1371/journal.pone.0110365
L-Endoglin overexpression increases renal fibrosis after unilateral ureteral obstruction
Abstract
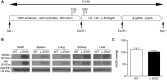
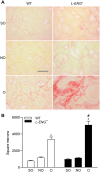
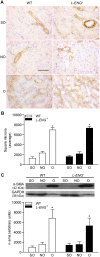
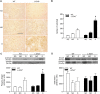
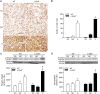
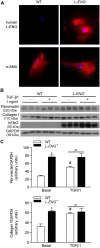
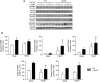
Transforming growth factor-β (TGF-β) plays a pivotal role in renal fibrosis. Endoglin, a 180 KDa membrane glycoprotein, is a TGF-β co-receptor overexpressed in several models of chronic kidney disease, but its function in renal fibrosis remains uncertain. Two membrane isoforms generated by alternative splicing have been described, L-Endoglin (long) and S-Endoglin (short) that differ from each other in their cytoplasmic tails, being L-Endoglin the most abundant isoform. The aim of this study was to assess the effect of L-Endoglin overexpression in renal tubulo-interstitial fibrosis. For this purpose, a transgenic mouse which ubiquitously overexpresses human L-Endoglin (L-ENG+) was generated and unilateral ureteral obstruction (UUO) was performed in L-ENG+ mice and their wild type (WT) littermates. Obstructed kidneys from L-ENG+ mice showed higher amounts of type I collagen and fibronectin but similar levels of α-smooth muscle actin (α-SMA) than obstructed kidneys from WT mice. Smad1 and Smad3 phosphorylation were significantly higher in obstructed kidneys from L-ENG+ than in WT mice. Our results suggest that the higher increase of renal fibrosis observed in L-ENG+ mice is not due to a major abundance of myofibroblasts, as similar levels of α-SMA were observed in both L-ENG+ and WT mice, but to the higher collagen and fibronectin synthesis by these fibroblasts. Furthermore, in vivo L-Endoglin overexpression potentiates Smad1 and Smad3 pathways and this effect is associated with higher renal fibrosis development.
Conflict of interest statement
Figures


References
-
- Grande MT, Lopez-Novoa JM (2009) Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328. - PubMed
-
- Border WA, Okuda S, Languino LR, Ruoslahti E (1990) Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int 37: 689–695. - PubMed
-
- Bitzer M, Sterzel RB, Bottinger EP (1998) Transforming growth factor-beta in renal disease. Kidney Blood Press Res 21: 1–12. - PubMed
-
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P (2005) TGF-beta receptor function in the endothelium. Cardiovasc Res 65: 599–608. - PubMed
-
- Moon JA, Kim HT, Cho IS, Sheen YY, Kim DK (2006) IN-1130, a novel transforming growth factor-beta type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int 70: 1234–1243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

