Timely inhibition of Notch signaling by DAPT promotes cardiac differentiation of murine pluripotent stem cells
- PMID: 25313563
- PMCID: PMC4196912
- DOI: 10.1371/journal.pone.0109588
Timely inhibition of Notch signaling by DAPT promotes cardiac differentiation of murine pluripotent stem cells
Abstract
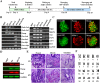
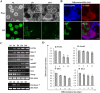
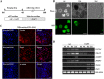
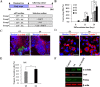
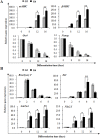
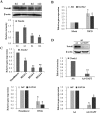
The Notch signaling pathway plays versatile roles during heart development. However, there is contradictory evidence that Notch pathway either facilitates or impairs cardiomyogenesis in vitro. In this study, we developed iPSCs by reprogramming of murine fibroblasts with GFP expression governed by Oct4 promoter, and identified an effective strategy to enhance cardiac differentiation through timely modulation of Notch signaling. The Notch inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester) alone drove the iPSCs to a neuronal fate. After mesoderm induction of embryoid bodies initiated by ascorbic acid (AA), the subsequent treatment of DAPT accelerated the generation of spontaneously beating cardiomyocytes. The timed synergy of AA and DAPT yielded an optimal efficiency of cardiac differentiation. Mechanistic studies showed that Notch pathway plays a biphasic role in cardiomyogenesis. It favors the early-stage cardiac differentiation, but exerts negative effects on the late-stage differentiation. Therefore, DAPT administration at the late stage enforced the inhibition of endogenous Notch activity, thereby enhancing cardiomyogenesis. In parallel, DAPT dramatically augmented the expression of Wnt3a, Wnt11, BMP2, and BMP4. In conclusion, our results highlight a practicable approach to generate cardiomyocytes from iPSCs based on the stage-specific biphasic roles of Notch signaling in cardiomyogenesis.
Conflict of interest statement
Figures

Similar articles
-
Knockdown of nucleosome assembly protein 1-like 1 induces mesoderm formation and cardiomyogenesis via notch signaling in murine-induced pluripotent stem cells.Stem Cells. 2014 Jul;32(7):1759-73. doi: 10.1002/stem.1702. Stem Cells. 2014. PMID: 24648372
-
Pharmacological inhibitor of notch signaling stabilizes the progression of small abdominal aortic aneurysm in a mouse model.J Am Heart Assoc. 2014 Oct 27;3(6):e001064. doi: 10.1161/JAHA.114.001064. J Am Heart Assoc. 2014. PMID: 25349182 Free PMC article.
-
NOTCH Signaling Is Essential for Maturation, Self-Renewal, and Tri-Differentiation of In Vitro Derived Human Neural Stem Cells.Cell Reprogram. 2017 Dec;19(6):372-383. doi: 10.1089/cell.2017.0009. Epub 2017 Oct 16. Cell Reprogram. 2017. PMID: 29035086
-
The Notch signaling pathway inhibitor Dapt alleviates autism-like behavior, autophagy and dendritic spine density abnormalities in a valproic acid-induced animal model of autism.Prog Neuropsychopharmacol Biol Psychiatry. 2019 Aug 30;94:109644. doi: 10.1016/j.pnpbp.2019.109644. Epub 2019 May 7. Prog Neuropsychopharmacol Biol Psychiatry. 2019. PMID: 31075347
-
The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling.Dev Dyn. 2007 Mar;236(3):886-92. doi: 10.1002/dvdy.21083. Dev Dyn. 2007. PMID: 17295317
Cited by
-
Inhibition of the NOTCH1 Pathway in the Stressed Heart Limits Fibrosis and Promotes Recruitment of Non-Myocyte Cells into the Cardiomyocyte Fate.J Cardiovasc Dev Dis. 2022 Apr 7;9(4):111. doi: 10.3390/jcdd9040111. J Cardiovasc Dev Dis. 2022. PMID: 35448087 Free PMC article.
-
The Potential of Gamma Secretase as a Therapeutic Target for Cardiac Diseases.J Pers Med. 2021 Dec 4;11(12):1294. doi: 10.3390/jpm11121294. J Pers Med. 2021. PMID: 34945766 Free PMC article. Review.
-
Induced pluripotent stem cell modelling of HLHS underlines the contribution of dysfunctional NOTCH signalling to impaired cardiogenesis.Hum Mol Genet. 2017 Aug 15;26(16):3031-3045. doi: 10.1093/hmg/ddx140. Hum Mol Genet. 2017. PMID: 28521042 Free PMC article.
-
Activation of AMPK promotes cardiac differentiation by stimulating the autophagy pathway.J Cell Commun Signal. 2023 Sep;17(3):939-955. doi: 10.1007/s12079-023-00744-z. Epub 2023 Apr 11. J Cell Commun Signal. 2023. PMID: 37040028 Free PMC article.
-
4-phenylbutyrate exerts stage-specific effects on cardiac differentiation via HDAC inhibition.PLoS One. 2021 Apr 21;16(4):e0250267. doi: 10.1371/journal.pone.0250267. eCollection 2021. PLoS One. 2021. PMID: 33882103 Free PMC article.
References
-
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920. - PubMed
-
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, et al. (2011) Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8: 228–240. - PubMed
-
- Kawai T, Takahashi T, Esaki M, Ushikoshi H, Nagano S, et al. (2004) Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J 68: 691–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

