TP53 mutational status is a potential marker for risk stratification in Wilms tumour with diffuse anaplasia
- PMID: 25313908
- PMCID: PMC4196953
- DOI: 10.1371/journal.pone.0109924
TP53 mutational status is a potential marker for risk stratification in Wilms tumour with diffuse anaplasia
Abstract
Purpose: The presence of diffuse anaplasia in Wilms tumours (DAWT) is associated with TP53 mutations and poor outcome. As patients receive intensified treatment, we sought to identify whether TP53 mutational status confers additional prognostic information.
Patients and methods: We studied 40 patients with DAWT with anaplasia in the tissue from which DNA was extracted and analysed for TP53 mutations and 17p loss. The majority of cases were profiled by copy number (n = 32) and gene expression (n = 36) arrays. TP53 mutational status was correlated with patient event-free and overall survival, genomic copy number instability and gene expression profiling.
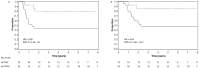
Results: From the 40 cases, 22 (55%) had TP53 mutations (2 detected only after deep-sequencing), 20 of which also had 17p loss (91%); 18 (45%) cases had no detectable mutation but three had 17p loss. Tumours with TP53 mutations and/or 17p loss (n = 25) had an increased risk of recurrence as a first event (p = 0.03, hazard ratio (HR), 3.89; 95% confidence interval (CI), 1.26-16.0) and death (p = 0.04, HR, 4.95; 95% CI, 1.36-31.7) compared to tumours lacking TP53 abnormalities. DAWT carrying TP53 mutations showed increased copy number alterations compared to those with wild-type, suggesting a more unstable genome (p = 0.03). These tumours showed deregulation of genes associated with cell cycle and DNA repair biological processes.
Conclusion: This study provides evidence that TP53 mutational analysis improves risk stratification in DAWT. This requires validation in an independent cohort before clinical use as a biomarker.
Conflict of interest statement
Figures
Similar articles
-
Significance of TP53 Mutation in Wilms Tumors with Diffuse Anaplasia: A Report from the Children's Oncology Group.Clin Cancer Res. 2016 Nov 15;22(22):5582-5591. doi: 10.1158/1078-0432.CCR-16-0985. Epub 2016 Oct 4. Clin Cancer Res. 2016. PMID: 27702824 Free PMC article.
-
Molecular profiling reveals frequent gain of MYCN and anaplasia-specific loss of 4q and 14q in Wilms tumor.Genes Chromosomes Cancer. 2011 Dec;50(12):982-95. doi: 10.1002/gcc.20907. Epub 2011 Aug 31. Genes Chromosomes Cancer. 2011. PMID: 21882282
-
Multiple mechanisms of MYCN dysregulation in Wilms tumour.Oncotarget. 2015 Mar 30;6(9):7232-43. doi: 10.18632/oncotarget.3377. Oncotarget. 2015. PMID: 25749049 Free PMC article.
-
Anaplasia in Wilms tumor: A critical review.Pediatr Blood Cancer. 2024 Jul;71(7):e31000. doi: 10.1002/pbc.31000. Epub 2024 Apr 11. Pediatr Blood Cancer. 2024. PMID: 38605554 Review.
-
The genetic changes of Wilms tumour.Nat Rev Nephrol. 2019 Apr;15(4):240-251. doi: 10.1038/s41581-019-0112-0. Nat Rev Nephrol. 2019. PMID: 30705419 Review.
Cited by
-
Identification of hub genes and construction of prognostic nomogram for patients with Wilms tumors.Front Oncol. 2022 Oct 21;12:982110. doi: 10.3389/fonc.2022.982110. eCollection 2022. Front Oncol. 2022. Retraction in: Front Oncol. 2023 Oct 26;13:1301668. doi: 10.3389/fonc.2023.1301668. PMID: 36338682 Free PMC article. Retracted.
-
Somatic TP53 Mutations Are Detectable in Circulating Tumor DNA from Children with Anaplastic Wilms Tumors.Transl Oncol. 2018 Dec;11(6):1301-1306. doi: 10.1016/j.tranon.2018.08.006. Epub 2018 Aug 29. Transl Oncol. 2018. PMID: 30172241 Free PMC article.
-
Delineating the interplay between oncogenic pathways and immunity in anaplastic Wilms tumors.Nat Commun. 2023 Nov 30;14(1):7884. doi: 10.1038/s41467-023-43290-3. Nat Commun. 2023. PMID: 38036539 Free PMC article.
-
Anaplastic histology Wilms' tumors registered to the Japan Wilms' Tumor Study Group are less aggressive than that in the National Wilms' Tumor Study 5.Pediatr Surg Int. 2016 Sep;32(9):851-5. doi: 10.1007/s00383-016-3929-7. Epub 2016 Jul 29. Pediatr Surg Int. 2016. PMID: 27473009
-
Efficacy analysis of multidisciplinary treatment for Wilms tumor in a single center.Pediatr Surg Int. 2023 Feb 27;39(1):141. doi: 10.1007/s00383-023-05408-y. Pediatr Surg Int. 2023. PMID: 36847869
References
-
- Green DM, Breslow NE, Beckwith JB, Finklestein JZ, Grundy P, et al. (1998) Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol 16: 3744–3751. - PubMed
-
- Green DM, Breslow NE, Beckwith JB, Ritchey ML, Shamberger RC, et al. (2001) Treatment with nephrectomy only for small, stage I/favorable histology Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol 19: 3719–3724. - PubMed
-
- Breslow NE, Ou SS, Beckwith JB, Haase GM, Kalapurakal JA, et al. (2004) Doxorubicin for favorable histology, Stage II-III Wilms tumor: results from the National Wilms Tumor Studies. Cancer 101: 1072–1080. - PubMed
-
- de Kraker J, Graf N, van Tinteren H, Pein F, Sandstedt B, et al. (2004) Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms' tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet 364: 1229–1235. - PubMed
-
- Pritchard-Jones K, Kelsey A, Vujanic G, Imeson J, Hutton C, et al. (2003) Older age is an adverse prognostic factor in stage I, favorable histology Wilms' tumor treated with vincristine monochemotherapy: a study by the United Kingdom Children's Cancer Study Group, Wilm's Tumor Working Group. J Clin Oncol 21: 3269–3275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

