Temporal and spatial regulation of epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function
- PMID: 25314967
- PMCID: PMC4226761
- DOI: 10.1126/scisignal.2005413
Temporal and spatial regulation of epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function
Abstract
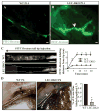
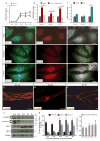
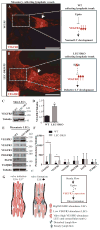
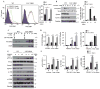
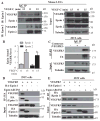
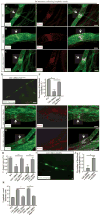
Lymphatic valves prevent the backflow of the lymph fluid and ensure proper lymphatic drainage throughout the body. Local accumulation of lymphatic fluid in tissues, a condition called lymphedema, is common in individuals with malformed lymphatic valves. The vascular endothelial growth factor receptor 3 (VEGFR3) is required for the development of lymphatic vascular system. The abundance of VEGFR3 in collecting lymphatic trunks is high before valve formation and, except at valve regions, decreases after valve formation. We found that in mesenteric lymphatics, the abundance of epsin 1 and 2, which are ubiquitin-binding adaptor proteins involved in endocytosis, was low at early stages of development. After lymphatic valve formation, the initiation of steady shear flow was associated with an increase in the abundance of epsin 1 and 2 in collecting lymphatic trunks, but not in valve regions. Epsin 1 and 2 bound to VEGFR3 and mediated the internalization and degradation of VEGFR3, resulting in termination of VEGFR3 signaling. Mice with lymphatic endothelial cell-specific deficiency of epsin 1 and 2 had dilated lymphatic capillaries, abnormally high VEGFR3 abundance in collecting lymphatics, immature lymphatic valves, and defective lymph drainage. Deletion of a single Vegfr3 allele or pharmacological suppression of VEGFR3 signaling restored normal lymphatic valve development and lymph drainage in epsin-deficient mice. Our findings establish a critical role for epsins in the temporal and spatial regulation of VEGFR3 abundance and signaling in collecting lymphatic trunks during lymphatic valve formation.
Copyright © 2014, American Association for the Advancement of Science.
Conflict of interest statement
Figures

References
-
- Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–97. - PubMed
-
- François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–7. - PubMed
-
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

