TNF-α alters the release and transfer of microparticle-encapsulated miRNAs from endothelial cells
- PMID: 25315114
- PMCID: PMC4233284
- DOI: 10.1152/physiolgenomics.00079.2014
TNF-α alters the release and transfer of microparticle-encapsulated miRNAs from endothelial cells
Abstract
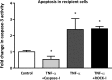
MicroRNAs (miRNAs) encapsulated within microparticles (MPs) are likely to have a role in cell-to-cell signaling in a variety of diseases, including atherosclerosis. However, little is known about the mechanisms by which different cell types release and transfer miRNAs. Here, we examined TNF-α-induced release of MP-encapsulated miR-126, miR-21, and miR-155 from human aortic endothelial cells (ECs) and their transfer to recipient cells. ECs were treated with TNF-α (100 ng/ml) in the presence or absence of inhibitors that target different MP production pathways. MPs released in response to TNF-α were characterized by: 1) 70-80% decrease in miRNA/MP levels for miR-126 and -21 but a significant increase in pre-miR-155 and miR-155 (P < 0.05), 2) 50% reduction in uptake by recipient cells (P < 0.05), and 3) diminished ability to transfer miRNA to recipient cells. Cotreatment of donor ECs with TNF-α and caspase inhibitor (Q-VD-OPH, 10 μM) produced MPs that had: 1) 1.5- to 2-fold increase in miRNA/MP loading, 2) enhanced uptake by recipient cells (2-fold), and 3) increased ability to transfer miR-155. Cotreatment of ECs with TNF-α and Rho-associated kinase (ROCK) inhibitor (10 μM) produced MPs with features similar to those produced by TNF-α treatment alone. Our data indicate that TNF-α induced the production of distinct MP populations: ROCK-dependent, miRNA-rich MPs that effectively transferred their cargo and were antiapoptotic, and caspase-dependent, miRNA-poor MPs that were proapoptotic. These data provide insight into the relationship between MP production and extracellular release of miRNA, as well as the potential of encapsulated miRNA for cell-to-cell communication.
Keywords: TNF-α; atherosclerosis; endothelial cells; extracellular RNA; miRNA release and transfer; microparticles.
Figures
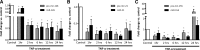




Similar articles
-
MicroRNA-19b functions as potential anti-thrombotic protector in patients with unstable angina by targeting tissue factor.J Mol Cell Cardiol. 2014 Oct;75:49-57. doi: 10.1016/j.yjmcc.2014.06.017. Epub 2014 Jul 3. J Mol Cell Cardiol. 2014. PMID: 24998411
-
Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism.J Cell Mol Med. 2015 Sep;19(9):2202-14. doi: 10.1111/jcmm.12607. Epub 2015 Jun 17. J Cell Mol Med. 2015. PMID: 26081516 Free PMC article.
-
Microparticles generated during chronic cerebral ischemia increase the permeability of microvascular endothelial barriers in vitro.Brain Res. 2016 Mar 1;1634:83-93. doi: 10.1016/j.brainres.2015.12.032. Epub 2015 Dec 23. Brain Res. 2016. PMID: 26723565
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
The clinical significance of platelet microparticle-associated microRNAs.Clin Chem Lab Med. 2017 May 1;55(5):657-666. doi: 10.1515/cclm-2016-0895. Clin Chem Lab Med. 2017. PMID: 28099120 Review.
Cited by
-
The Impact of microRNAs in Renin-Angiotensin-System-Induced Cardiac Remodelling.Int J Mol Sci. 2021 Apr 30;22(9):4762. doi: 10.3390/ijms22094762. Int J Mol Sci. 2021. PMID: 33946230 Free PMC article. Review.
-
The role of microvesicles containing microRNAs in vascular endothelial dysfunction.J Cell Mol Med. 2019 Dec;23(12):7933-7945. doi: 10.1111/jcmm.14716. Epub 2019 Oct 1. J Cell Mol Med. 2019. PMID: 31576661 Free PMC article. Review.
-
Resistin and Cardiac Arrest-A Prospective Study.J Clin Med. 2019 Dec 25;9(1):57. doi: 10.3390/jcm9010057. J Clin Med. 2019. PMID: 31881807 Free PMC article.
-
Microparticles: biogenesis, characteristics and intervention therapy for cancers in preclinical and clinical research.J Nanobiotechnology. 2022 Apr 13;20(1):189. doi: 10.1186/s12951-022-01358-0. J Nanobiotechnology. 2022. PMID: 35418077 Free PMC article. Review.
-
Interleukin-32α Inhibits Endothelial Inflammation, Vascular Smooth Muscle Cell Activation, and Atherosclerosis by Upregulating Timp3 and Reck through suppressing microRNA-205 Biogenesis.Theranostics. 2017 Jun 1;7(8):2186-2203. doi: 10.7150/thno.18407. eCollection 2017. Theranostics. 2017. PMID: 28740544 Free PMC article.
References
-
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108: 5003–5008, 2011. - PMC - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004. - PubMed
-
- Bouchareb R, Boulanger MC, Fournier D, Pibarot P, Messaddeq Y, Mathieu P. Mechanical strain induces the production of spheroid mineralized microparticles in the aortic valve through a RhoA/ROCK-dependent mechanism. J Mol Cell Cardiol 67: 49–59, 2014. - PubMed
-
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010. - PubMed
-
- Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8: 345–352, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

