Autoimmune encephalopathies
- PMID: 25315420
- PMCID: PMC4363225
- DOI: 10.1111/nyas.12553
Autoimmune encephalopathies
Abstract
Over the past 10 years, the continual discovery of novel forms of encephalitis associated with antibodies to cell-surface or synaptic proteins has changed the paradigms for diagnosing and treating disorders that were previously unknown or mischaracterized. We review here the process of discovery, the symptoms, and the target antigens of 11 autoimmune encephalitic disorders, grouped by syndromes and approached from a clinical perspective. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis, several subtypes of limbic encephalitis, stiff-person spectrum disorders, and other autoimmune encephalitides that result in psychosis, seizures, or abnormal movements are described in detail. We include a novel encephalopathy with prominent sleep dysfunction that provides an intriguing link between chronic neurodegeneration and cell-surface autoimmunity (IgLON5). Some of the caveats of limited serum testing are outlined. In addition, we review the underlying cellular and synaptic mechanisms that for some disorders confirm the antibody pathogenicity. The multidisciplinary impact of autoimmune encephalitis has been expanded recently by the discovery that herpes simplex encephalitis is a robust trigger of synaptic autoimmunity, and that some patients may develop overlapping syndromes, including anti-NMDAR encephalitis and neuromyelitis optica or other demyelinating diseases.
Keywords: anti-NMDAR antibodies; autoimmune encephalitis; limbic encephalitis; psychosis; treatment.
© 2014 New York Academy of Sciences.
Figures

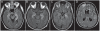


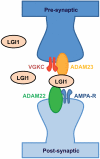


References
-
- Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol. 2011;10:1098–1107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

