Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis
- PMID: 25315431
- PMCID: PMC4259248
- DOI: 10.1158/1940-6207.CAPR-14-0182
Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis
Abstract
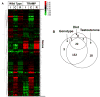
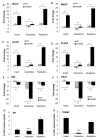
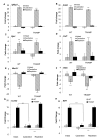
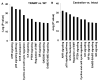
Consumption of tomato products containing the carotenoid lycopene is associated with a reduced risk of prostate cancer. To identify gene expression patterns associated with early testosterone-driven prostate carcinogenesis, which are impacted by dietary tomato and lycopene, wild-type (WT) and transgenic adenocarcinoma of the mouse prostate (TRAMP) mice were fed control or tomato- or lycopene-containing diets from 4 to 10 weeks of age. Eight-week-old mice underwent sham surgery, castration, or castration followed by testosterone repletion (2.5 mg/kg/d initiated 1 week after castration). Ten-week-old intact TRAMP mice exhibit early multifocal prostatic intraepithelial neoplasia. Of the 200 prostate cancer-related genes measured by quantitative NanoString, 189 are detectable, 164 significantly differ by genotype, 179 by testosterone status, and 30 by diet type (P < 0.05). In TRAMP, expression of Birc5, Mki67, Aurkb, Ccnb2, Foxm1, and Ccne2 is greater compared with WT and is decreased by castration. In parallel, castration reduces Ki67-positive staining (P < 0.0001) compared with intact and testosterone-repleted TRAMP mice. Expression of genes involved in androgen metabolism/signaling pathways is reduced by lycopene feeding (Srd5a1) and by tomato feeding (Srd5a2, Pxn, and Srebf1). In addition, tomato feeding significantly reduced expression of genes associated with stem cell features, Aldh1a and Ly6a, whereas lycopene feeding significantly reduced expression of neuroendocrine differentiation-related genes, Ngfr and Syp. Collectively, these studies demonstrate a profile of testosterone-regulated genes associated with early prostate carcinogenesis that are potential mechanistic targets of dietary tomato components. Future studies on androgen signaling/metabolism, stem cell features, and neuroendocrine differentiation pathways may elucidate the mechanisms by which dietary tomato and lycopene impact prostate cancer risk.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures

Similar articles
-
Orchestration of miRNA Patterns by Testosterone and Dietary Tomato Carotenoids during Early Prostate Carcinogenesis in TRAMP Mice.J Nutr. 2023 Jul;153(7):1877-1888. doi: 10.1016/j.tjnut.2023.05.015. Epub 2023 May 13. J Nutr. 2023. PMID: 37187350 Free PMC article.
-
β-Carotene Oxygenase 2 Genotype Modulates the Impact of Dietary Lycopene on Gene Expression during Early TRAMP Prostate Carcinogenesis.J Nutr. 2022 Apr 1;152(4):950-960. doi: 10.1093/jn/nxab445. J Nutr. 2022. PMID: 34964896 Free PMC article.
-
β-Carotene 9',10' Oxygenase Modulates the Anticancer Activity of Dietary Tomato or Lycopene on Prostate Carcinogenesis in the TRAMP Model.Cancer Prev Res (Phila). 2017 Feb;10(2):161-169. doi: 10.1158/1940-6207.CAPR-15-0402. Epub 2016 Nov 2. Cancer Prev Res (Phila). 2017. PMID: 27807077 Free PMC article.
-
Tomatoes, Lycopene, and Prostate Cancer: What Have We Learned from Experimental Models?J Nutr. 2022 Jun 9;152(6):1381-1403. doi: 10.1093/jn/nxac066. J Nutr. 2022. PMID: 35278075 Free PMC article. Review.
-
Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials.Eur J Clin Nutr. 2007 Mar;61(3):295-303. doi: 10.1038/sj.ejcn.1602510. Epub 2006 Aug 16. Eur J Clin Nutr. 2007. PMID: 16929242 Review.
Cited by
-
Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids.Adv Nutr. 2018 Jul 1;9(4):465-492. doi: 10.1093/advances/nmy025. Adv Nutr. 2018. PMID: 30032230 Free PMC article. Review.
-
Can Lycopene Impact the Androgen Axis in Prostate Cancer?: A Systematic Review of Cell Culture and Animal Studies.Nutrients. 2019 Mar 15;11(3):633. doi: 10.3390/nu11030633. Nutrients. 2019. PMID: 30875962 Free PMC article.
-
Orchestration of miRNA Patterns by Testosterone and Dietary Tomato Carotenoids during Early Prostate Carcinogenesis in TRAMP Mice.J Nutr. 2023 Jul;153(7):1877-1888. doi: 10.1016/j.tjnut.2023.05.015. Epub 2023 May 13. J Nutr. 2023. PMID: 37187350 Free PMC article.
-
Role of Vitamins in Therapeutic and Targeting Approaches for Prostate Cancer: An Overview.Curr Drug Targets. 2024;25(14):934-952. doi: 10.2174/0113894501314558240822082557. Curr Drug Targets. 2024. PMID: 39257155 Review.
-
Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines.Foods. 2019 Jun 10;8(6):201. doi: 10.3390/foods8060201. Foods. 2019. PMID: 31185698 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

