PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper
- PMID: 25315681
- PMCID: PMC4394022
- DOI: 10.1016/j.nbd.2014.10.002
PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper
Abstract
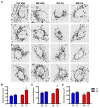
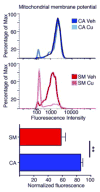
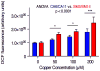
Poorly-defined interactions between environmental and genetic risk factors underlie Parkinson's disease (PD) etiology. Here we tested the hypothesis that human stem cell derived forebrain neuroprogenitors from patients with known familial risk for early onset PD will exhibit enhanced sensitivity to PD environmental risk factors compared to healthy control subjects without a family history of PD. Two male siblings (SM and PM) with biallelic loss-of-function mutations in PARK2 were identified. Human induced pluripotent stem cells (hiPSCs) from SM, PM, and four control subjects with no known family histories of PD or related neurodegenerative diseases were utilized. We tested the hypothesis that hiPSC-derived neuroprogenitors from patients with PARK2 mutations would show heightened cell death, mitochondrial dysfunction, and reactive oxygen species generation compared to control cells as a result of exposure to heavy metals (PD environmental risk factors). We report that PARK2 mutant neuroprogenitors showed increased cytotoxicity with copper (Cu) and cadmium (Cd) exposure but not manganese (Mn) or methyl mercury (MeHg) relative to control neuroprogenitors. PARK2 mutant neuroprogenitors also showed a substantial increase in mitochondrial fragmentation, initial ROS generation, and loss of mitochondrial membrane potential following Cu exposure. Our data substantiate Cu exposure as an environmental risk factor for PD. Furthermore, we report a shift in the lowest observable effect level (LOEL) for greater sensitivity to Cu-dependent mitochondrial dysfunction in patients SM and PM relative to controls, correlating with their increased genetic risk for PD.
Keywords: Copper; Environmental risk factors; Neurotoxicty; PARK2; Parkinson's disease.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




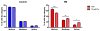
References
-
- Abbas N, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson’s Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson’s Disease. Hum Mol Genet. 1999;8:567–74. - PubMed
-
- Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6. - PubMed
-
- Boll M-C, et al. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33:1717–23. - PubMed
-
- Buzanska L, et al. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells. 2009;27:2591–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES000267/ES/NIEHS NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- K02NS057666/NS/NINDS NIH HHS/United States
- P01 GM085354/GM/NIGMS NIH HHS/United States
- ES016931-02S1/ES/NIEHS NIH HHS/United States
- RR166-737/4787736/RR/NCRR NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- R01 NS078289/NS/NINDS NIH HHS/United States
- K02 NS057666/NS/NINDS NIH HHS/United States
- R01 ES010563/ES/NIEHS NIH HHS/United States
- 1 RO1 NS078289/NS/NINDS NIH HHS/United States
- F31 NS077632/NS/NINDS NIH HHS/United States
- R01 HL104040/HL/NHLBI NIH HHS/United States
- T32 GM07347/GM/NIGMS NIH HHS/United States
- 5P30 ES000267/ES/NIEHS NIH HHS/United States
- 5P01 GM08535403/GM/NIGMS NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- P30HD15052/HD/NICHD NIH HHS/United States
- R01 ES016931/ES/NIEHS NIH HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- ES016931/ES/NIEHS NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

