Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer
- PMID: 25315765
- PMCID: PMC4145231
- DOI: 10.1186/s13059-014-0428-9
Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer
Abstract
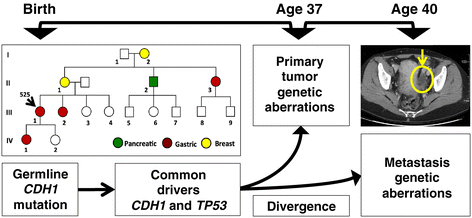
Background: Gastric cancer is the second-leading cause of global cancer deaths, with metastatic disease representing the primary cause of mortality. To identify candidate drivers involved in oncogenesis and tumor evolution, we conduct an extensive genome sequencing analysis of metastatic progression in a diffuse gastric cancer. This involves a comparison between a primary tumor from a hereditary diffuse gastric cancer syndrome proband and its recurrence as an ovarian metastasis.
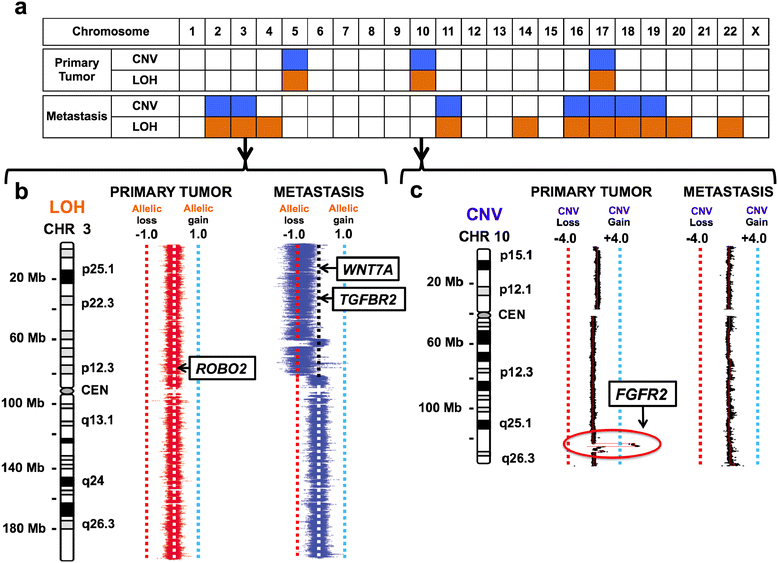
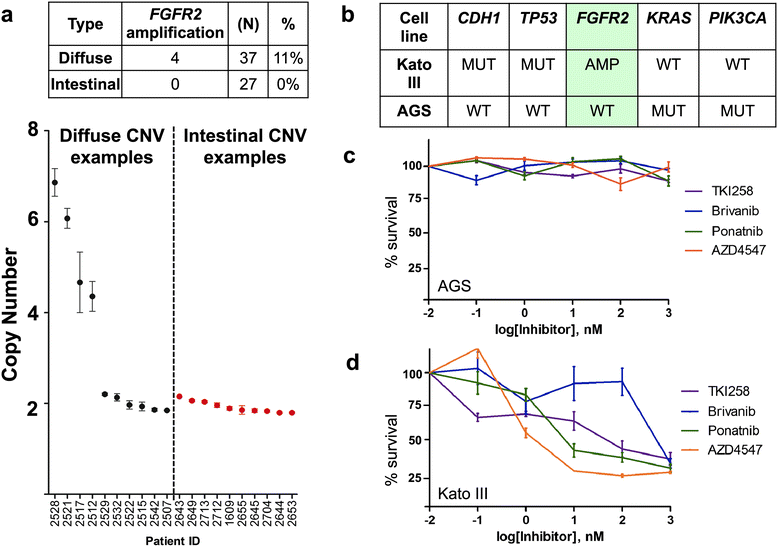
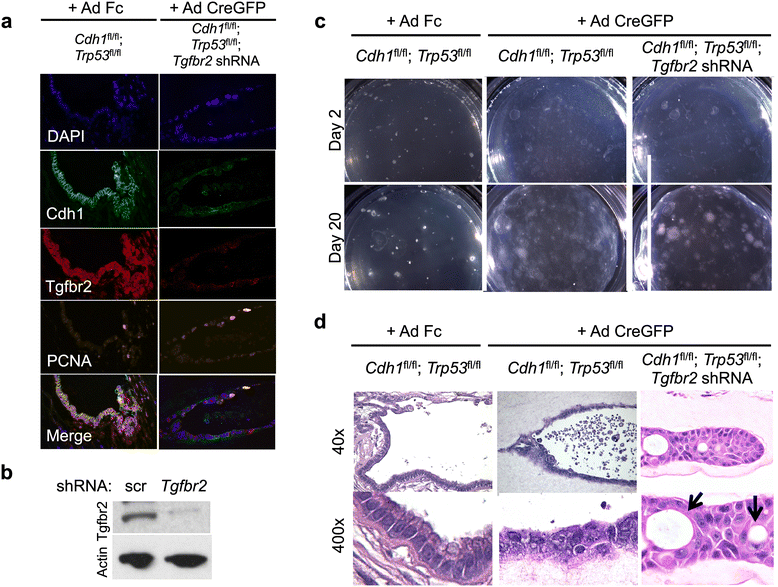
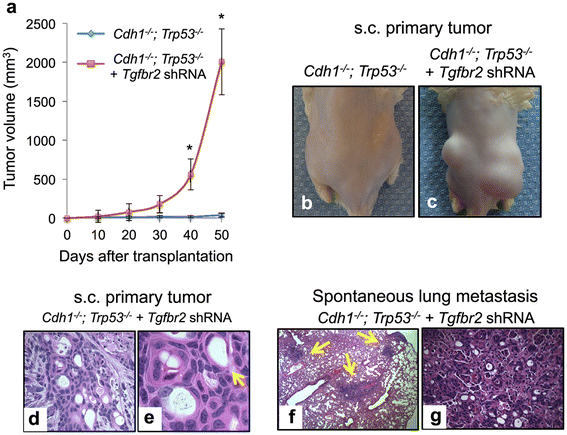
Results: Both the primary tumor and ovarian metastasis have common biallelic loss-of-function of both the CDH1 and TP53 tumor suppressors, indicating a common genetic origin. While the primary tumor exhibits amplification of the Fibroblast growth factor receptor 2 (FGFR2) gene, the metastasis notably lacks FGFR2 amplification but rather possesses unique biallelic alterations of Transforming growth factor-beta receptor 2 (TGFBR2), indicating the divergent in vivo evolution of a TGFBR2-mutant metastatic clonal population in this patient. As TGFBR2 mutations have not previously been functionally validated in gastric cancer, we modeled the metastatic potential of TGFBR2 loss in a murine three-dimensional primary gastric organoid culture. The Tgfbr2 shRNA knockdown within Cdh1-/-; Tp53-/- organoids generates invasion in vitro and robust metastatic tumorigenicity in vivo, confirming Tgfbr2 metastasis suppressor activity.
Conclusions: We document the metastatic differentiation and genetic heterogeneity of diffuse gastric cancer and reveal the potential metastatic role of TGFBR2 loss-of-function. In support of this study, we apply a murine primary organoid culture method capable of recapitulating in vivo metastatic gastric cancer. Overall, we describe an integrated approach to identify and functionally validate putative cancer drivers involved in metastasis.
Figures

References
-
- Lauren P. Histogenesis of intestinal and diffuse types of gastric carcinoma. Scand J Gastroenterol Suppl. 1991;180:160–164. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. - DOI - PubMed
-
- Lee YS, Cho YS, Lee GK, Lee S, Kim YW, Jho S, Kim HM, Hong SH, Hwang JA, Kim SY, Hong D, Choi IJ, Kim BC, Kim BC, Kim CH, Choi H, Kim Y, Kim KW, Kong G, Kim HL, Bhak J, Lee SH, Lee JS. Genomic profile analysis of diffuse-type gastric cancers. Genome Biol. 2014;15:R55. doi: 10.1186/gb-2014-15-4-r55. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HG006137/HG/NHGRI NIH HHS/United States
- U01 CA176299/CA/NCI NIH HHS/United States
- T32 CA121940/CA/NCI NIH HHS/United States
- K08 CA166512/CA/NCI NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- U01 DK085527/DK/NIDDK NIH HHS/United States
- DK56339/DK/NIDDK NIH HHS/United States
- U01CA17629901/CA/NCI NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- R33 CA174575/CA/NCI NIH HHS/United States
- P30 DK056339/DK/NIDDK NIH HHS/United States
- HG005570/HG/NHGRI NIH HHS/United States
- 1U01DK085527/DK/NIDDK NIH HHS/United States
- RC2 HG005570/HG/NHGRI NIH HHS/United States
- U01 CA151920/CA/NCI NIH HHS/United States
- 1R33CA1745701/CA/NCI NIH HHS/United States
- 1U01CA151920/CA/NCI NIH HHS/United States
- P01 HG000205/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

