Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5
- PMID: 25315771
- PMCID: PMC4256320
- DOI: 10.1074/jbc.M114.608919
Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5
Abstract
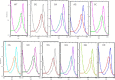
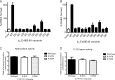
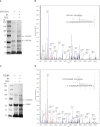
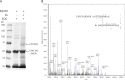
Two acidic residues, Glu-48 and Glu-49, of cytochrome b5 (b5) are essential for stimulating the 17,20-lyase activity of cytochrome P450c17 (CYP17A1). Substitution of Ala, Gly, Cys, or Gln for these two glutamic acid residues abrogated all capacity to stimulate 17,20-lyase activity. Mutations E49D and E48D/E49D retained 23 and 38% of wild-type activity, respectively. Using the zero-length cross-linker ethyl-3-(3-dimethylaminopropyl)carbodiimide, we obtained cross-linked heterodimers of b5 and CYP17A1, wild-type, or mutations R347K and R358K. In sharp contrast, the b5 double mutation E48G/E49G did not form cross-linked complexes with wild-type CYP17A1. Mass spectrometric analysis of the CYP17A1-b5 complexes identified two cross-linked peptide pairs as follows: CYP17A1-WT: (84)EVLIKK(89)-b5: (53)EQAGGDATENFEDVGHSTDAR(73) and CYP17A1-R347K: (341)TPTISDKNR(349)-b5: (40)FLEEHPGGEEVLR(52). Using these two sites of interaction and Glu-48/Glu-49 in b5 as constraints, protein docking calculations based on the crystal structures of the two proteins yielded a structural model of the CYP17A1-b5 complex. The appositional surfaces include Lys-88, Arg-347, and Arg-358/Arg-449 of CYP17A1, which interact with Glu-61, Glu-42, and Glu-48/Glu-49 of b5, respectively. Our data reveal the structural basis of the electrostatic interactions between these two proteins, which is critical for 17,20-lyase activity and androgen biosynthesis.
Keywords: Allosteric Regulation; Androgen; CYP17A1; Cytochrome P450; Cytochrome b5; Mass Spectrometry (MS); Protein Cross-linking; Steroidogenesis.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Attard G., Reid A. H., Yap T. A., Raynaud F., Dowsett M., Settatree S., Barrett M., Parker C., Martins V., Folkerd E., Clark J., Cooper C. S., Kaye S. B., Dearnaley D., Lee G., de Bono J. S. (2008) Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 - PubMed
-
- Miller W. L., Auchus R. J., Geller D. H. (1997) The regulation of 17,20 lyase activity. Steroids 62, 133–142 - PubMed
-
- Auchus R. J., Lee T. C., Miller W. L. (1998) Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J. Biol. Chem. 273, 3158–3165 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

