Phosphorylation of threonine 3 on histone H3 by haspin kinase is required for meiosis I in mouse oocytes
- PMID: 25315835
- PMCID: PMC4248095
- DOI: 10.1242/jcs.158840
Phosphorylation of threonine 3 on histone H3 by haspin kinase is required for meiosis I in mouse oocytes
Abstract
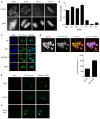
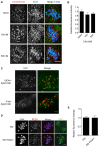
Meiosis I (MI), the division that generates haploids, is prone to errors that lead to aneuploidy in females. Haspin is a kinase that phosphorylates histone H3 on threonine 3, thereby recruiting Aurora kinase B (AURKB) and the chromosomal passenger complex (CPC) to kinetochores to regulate mitosis. Haspin and AURKC, an AURKB homolog, are enriched in germ cells, yet their significance in regulating MI is not fully understood. Using inhibitors and overexpression approaches, we show a role for haspin during MI in mouse oocytes. Haspin-perturbed oocytes display abnormalities in chromosome morphology and alignment, improper kinetochore-microtubule attachments at metaphase I and aneuploidy at metaphase II. Unlike in mitosis, kinetochore localization remained intact, whereas the distribution of the CPC along chromosomes was absent. The meiotic defects following haspin inhibition were similar to those observed in oocytes where AURKC was inhibited, suggesting that the correction of microtubule attachments during MI requires AURKC along chromosome arms rather than at kinetochores. Our data implicate haspin as a regulator of the CPC and chromosome segregation during MI, while highlighting important differences in how chromosome segregation is regulated between MI and mitosis.
Keywords: Aurora kinase; Haspin kinase; Histone phosphorylation; Meiosis I; Meiotic maturation; Oocyte.
© 2014. Published by The Company of Biologists Ltd.
Figures




Similar articles
-
Haspin inhibition reveals functional differences of interchromatid axis-localized AURKB and AURKC.Mol Biol Cell. 2017 Aug 15;28(17):2233-2240. doi: 10.1091/mbc.E16-12-0850. Epub 2017 Jun 28. Mol Biol Cell. 2017. PMID: 28659416 Free PMC article.
-
Chromosome segregation regulation in human zygotes: altered mitotic histone phosphorylation dynamics underlying centromeric targeting of the chromosomal passenger complex.Hum Reprod. 2015 Oct;30(10):2275-91. doi: 10.1093/humrep/dev186. Epub 2015 Jul 29. Hum Reprod. 2015. PMID: 26223676
-
Haspin kinase regulates microtubule-organizing center clustering and stability through Aurora kinase C in mouse oocytes.J Cell Sci. 2016 Oct 1;129(19):3648-3660. doi: 10.1242/jcs.189340. Epub 2016 Aug 25. J Cell Sci. 2016. PMID: 27562071 Free PMC article.
-
Establishing correct kinetochore-microtubule attachments in mitosis and meiosis.Essays Biochem. 2020 Sep 4;64(2):277-287. doi: 10.1042/EBC20190072. Essays Biochem. 2020. PMID: 32406497 Free PMC article. Review.
-
Haspin: a newly discovered regulator of mitotic chromosome behavior.Chromosoma. 2010 Apr;119(2):137-47. doi: 10.1007/s00412-009-0250-4. Epub 2009 Dec 8. Chromosoma. 2010. PMID: 19997740 Free PMC article. Review.
Cited by
-
Aurora B and Aurora C pools at two chromosomal regions collaboratively maintain chromosome alignment and prevent aneuploidy at the second meiotic division in mammalian oocytes.Front Cell Dev Biol. 2024 Sep 17;12:1470981. doi: 10.3389/fcell.2024.1470981. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39355122 Free PMC article.
-
A Haspin promoter element induces tissue-specific methylation of a transcription region and the regulation of gene expression in mouse ova.Cell J. 2022 Sep 12;24(9):552-554. doi: 10.22074/cellj.2022.8444. Cell J. 2022. PMID: 36274209 Free PMC article.
-
Histone H3 N-Terminal Tail Residues Important for Meiosis in Saccharomyces cerevisiae.Biomolecules. 2025 Aug 21;15(8):1202. doi: 10.3390/biom15081202. Biomolecules. 2025. PMID: 40867646 Free PMC article.
-
RBBP4 regulates histone deacetylation and bipolar spindle assembly during oocyte maturation in the mouse.Biol Reprod. 2015 Apr;92(4):105. doi: 10.1095/biolreprod.115.128298. Epub 2015 Mar 18. Biol Reprod. 2015. PMID: 25788661 Free PMC article.
-
Histone H3 tail modifications required for meiosis in Saccharomyces cerevisiae.bioRxiv [Preprint]. 2024 Dec 11:2024.12.09.627563. doi: 10.1101/2024.12.09.627563. bioRxiv. 2024. PMID: 39713340 Free PMC article. Preprint.
References
-
- Ashtiyani R. K., Moghaddam A. M., Schubert V., Rutten T., Fuchs J., Demidov D., Blattner F. R., Houben A. (2011). AtHaspin phosphorylates histone H3 at threonine 3 during mitosis and contributes to embryonic patterning in Arabidopsis. Plant J. 68, 443–454 10.1111/j.1365-313X.2011.04699.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

