Anesthetics target interfacial transmembrane sites in nicotinic acetylcholine receptors
- PMID: 25316107
- PMCID: PMC4394016
- DOI: 10.1016/j.neuropharm.2014.10.002
Anesthetics target interfacial transmembrane sites in nicotinic acetylcholine receptors
Abstract
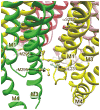
General anesthetics are a heterogeneous group of small amphiphilic ligands that interact weakly at multiple allosteric sites on many pentameric ligand gated ion channels (pLGICs), resulting in either inhibition, potentiation of channel activity, or both. Allosteric principles imply that modulator sites must change configuration and ligand affinity during receptor state transitions. Thus, general anesthetics and related compounds are useful both as state-dependent probes of receptor structure and as potentially selective modulators of pLGIC functions. This review focuses on general anesthetic sites in nicotinic acetylcholine receptors, which were among the first anesthetic-sensitive pLGIC experimental models studied, with particular focus on sites formed by transmembrane domain elements. Structural models place many of these sites at interfaces between two or more pLGIC transmembrane helices both within subunits and between adjacent subunits, and between transmembrane helices and either lipids (the lipid-protein interface) or water (i.e. the ion channel). A single general anesthetic may bind at multiple allosteric sites in pLGICs, producing a net effect of either inhibition (e.g. blocking the ion channel) or enhanced channel gating (e.g. inter-subunit sites). Other general anesthetic sites identified by photolabeling or crystallography are tentatively linked to functional effects, including intra-subunit helix bundle sites and the lipid-protein interface. This article is part of the Special Issue entitled 'The Nicotinic Acetylcholine Receptor: From Molecular Biology to Cognition'.
Keywords: Alcohol; Allosterism; Barbiturates; Mutagenesis; Photolabel; Propofol.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


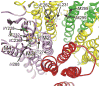
Similar articles
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
Multiple transmembrane binding sites for p-trifluoromethyldiazirinyl-etomidate, a photoreactive Torpedo nicotinic acetylcholine receptor allosteric inhibitor.J Biol Chem. 2011 Jun 10;286(23):20466-77. doi: 10.1074/jbc.M111.219071. Epub 2011 Apr 15. J Biol Chem. 2011. PMID: 21498509 Free PMC article.
-
The nicotinic acetylcholine receptor and its prokaryotic homologues: Structure, conformational transitions & allosteric modulation.Neuropharmacology. 2015 Sep;96(Pt B):137-49. doi: 10.1016/j.neuropharm.2014.12.006. Epub 2014 Dec 18. Neuropharmacology. 2015. PMID: 25529272 Review.
-
Allosteric modulators of the α4β2 subtype of neuronal nicotinic acetylcholine receptors.Biochem Pharmacol. 2011 Oct 15;82(8):952-8. doi: 10.1016/j.bcp.2011.04.020. Epub 2011 May 7. Biochem Pharmacol. 2011. PMID: 21596025 Free PMC article. Review.
-
Allosteric modulation of nicotinic acetylcholine receptors.Biochem Pharmacol. 2015 Oct 15;97(4):408-417. doi: 10.1016/j.bcp.2015.07.028. Epub 2015 Jul 29. Biochem Pharmacol. 2015. PMID: 26231943 Review.
Cited by
-
Modulation of a rapid neurotransmitter receptor-ion channel by membrane lipids.Front Cell Dev Biol. 2024 Jan 11;11:1328875. doi: 10.3389/fcell.2023.1328875. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38274273 Free PMC article. Review.
-
High Throughput Screen for Escherichia coli Twin Arginine Translocation (Tat) Inhibitors.PLoS One. 2016 Feb 22;11(2):e0149659. doi: 10.1371/journal.pone.0149659. eCollection 2016. PLoS One. 2016. PMID: 26901445 Free PMC article.
-
Permeating disciplines: Overcoming barriers between molecular simulations and classical structure-function approaches in biological ion transport.Biochim Biophys Acta Biomembr. 2018 Apr;1860(4):927-942. doi: 10.1016/j.bbamem.2017.12.013. Epub 2017 Dec 16. Biochim Biophys Acta Biomembr. 2018. PMID: 29258839 Free PMC article. Review.
-
Ion Channels in Anesthesia.Adv Exp Med Biol. 2021;1349:401-413. doi: 10.1007/978-981-16-4254-8_19. Adv Exp Med Biol. 2021. PMID: 35138625
-
Allosteric potentiation of a ligand-gated ion channel is mediated by access to a deep membrane-facing cavity.Proc Natl Acad Sci U S A. 2018 Oct 16;115(42):10672-10677. doi: 10.1073/pnas.1809650115. Epub 2018 Oct 1. Proc Natl Acad Sci U S A. 2018. PMID: 30275330 Free PMC article.
References
-
- Abadji VC, Raines DE, Watts A, Miller KW. The effect of general anesthetics on the dynamics of phosphatidylcholine-acetylcholine receptor interactions in reconstituted vesicles. Biochim Biophys Acta. 1993;1147:143–153. - PubMed
-
- Absalom NL, Lewis TM, Kaplan W, Pierce KD, Schofield PR. Role of charged residues in coupling ligand binding and channel activation in the extracellular domain of the glycine receptor. J Biol Chem. 2003;278:50151–50157. - PubMed
-
- Addona GH, Kloczewiak MA, Miller KW. Time-resolved photolabeling of membrane proteins: Application to the nicotinic acetylcholine receptor. Anal Biochem. 1999;267:135–140. - PubMed
-
- Aistrup GL, Marszalec W, Narahashi T. Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol. 1999;55:39–49. - PubMed
-
- Akabas MH, Karlin A. Identification of acetylcholine receptor channel-lining residues in the M1 segment of the alpha-subunit. Biochemistry. 1995;34:12496–12500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

