Multiple Brain Markers are Linked to Age-Related Variation in Cognition
- PMID: 25316342
- PMCID: PMC4785941
- DOI: 10.1093/cercor/bhu238
Multiple Brain Markers are Linked to Age-Related Variation in Cognition
Abstract
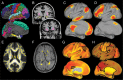
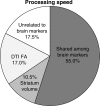
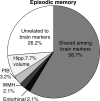
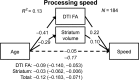
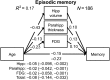
Age-related alterations in brain structure and function have been challenging to link to cognition due to potential overlapping influences of multiple neurobiological cascades. We examined multiple brain markers associated with age-related variation in cognition. Clinically normal older humans aged 65-90 from the Harvard Aging Brain Study (N = 186) were characterized on a priori magnetic resonance imaging markers of gray matter thickness and volume, white matter hyperintensities, fractional anisotropy (FA), resting-state functional connectivity, positron emission tomography markers of glucose metabolism and amyloid burden, and cognitive factors of processing speed, executive function, and episodic memory. Partial correlation and mediation analyses estimated age-related variance in cognition shared with individual brain markers and unique to each marker. The largest relationships linked FA and striatum volume to processing speed and executive function, and hippocampal volume to episodic memory. Of the age-related variance in cognition, 70-80% was accounted for by combining all brain markers (but only ∼20% of total variance). Age had significant indirect effects on cognition via brain markers, with significant markers varying across cognitive domains. These results suggest that most age-related variation in cognition is shared among multiple brain markers, but potential specificity between some brain markers and cognitive domains motivates additional study of age-related markers of neural health.
Keywords: aging; amyloid; executive function; memory; white matter.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. 2006. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 66:1837–1844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

