Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS
- PMID: 25316679
- PMCID: PMC4263989
- DOI: 10.1182/blood-2014-07-587477
Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS
Abstract
High-dose, posttransplantation cyclophosphamide (PTCy) reduces severe graft-versus-host disease (GVHD) after allogeneic blood or marrow transplantation (alloBMT), but the impact of PTCy on long-term, disease-specific outcomes is unclear. We conducted a retrospective study of 209 consecutive adult patients transplanted for acute myeloid leukemia (AML, n = 138), myelodysplastic syndrome (n = 28), or acute lymphoblastic leukemia (ALL, n = 43) using PTCy as sole GVHD prophylaxis after myeloablative conditioning and HLA-matched-related or -unrelated T-cell-replete allografting. At alloBMT, 30% of patients were not in morphologic complete remission. The cumulative incidences of grades II to IV and III to IV acute GVHD at 100 days and chronic GVHD at 2 years were 45%, 11%, and 13%, respectively. Forty-three percent of patients did not require immunosuppression for any reason beyond PTCy. At 3 years, relapse cumulative incidence was 36%, disease-free survival was 46%, survival free of disease and chronic GVHD was 39%, and overall survival was 58%. Lack of remission at alloBMT, adverse cytogenetics, and low allograft nucleated cell dose were associated with inferior survival for AML patients. Minimal residual disease but not t(9;22) was associated with inferior outcomes for ALL patients. The ability to limit posttransplantation immunosuppression makes PTCy a promising transplantation platform for the integration of postgrafting strategies to prevent relapse.
© 2014 by The American Society of Hematology.
Figures
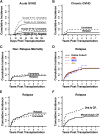

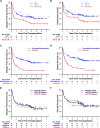
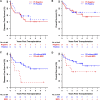
Comment in
-
GVHD prophylaxis made safe, easy, and inexpensive.Blood. 2014 Dec 11;124(25):3672-3. doi: 10.1182/blood-2014-10-607879. Blood. 2014. PMID: 25498453
References
-
- Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529–1533. - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. - PubMed
-
- Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

