LIMP-2 expression is critical for β-glucocerebrosidase activity and α-synuclein clearance
- PMID: 25316793
- PMCID: PMC4217458
- DOI: 10.1073/pnas.1405700111
LIMP-2 expression is critical for β-glucocerebrosidase activity and α-synuclein clearance
Abstract
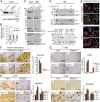
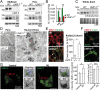
Mutations within the lysosomal enzyme β-glucocerebrosidase (GC) result in Gaucher disease and represent a major risk factor for developing Parkinson disease (PD). Loss of GC activity leads to accumulation of its substrate glucosylceramide and α-synuclein. Since lysosomal activity of GC is tightly linked to expression of its trafficking receptor, the lysosomal integral membrane protein type-2 (LIMP-2), we studied α-synuclein metabolism in LIMP-2-deficient mice. These mice showed an α-synuclein dosage-dependent phenotype, including severe neurological impairments and premature death. In LIMP-2-deficient brains a significant reduction in GC activity led to lipid storage, disturbed autophagic/lysosomal function, and α-synuclein accumulation mediating neurotoxicity of dopaminergic (DA) neurons, apoptotic cell death, and inflammation. Heterologous expression of LIMP-2 accelerated clearance of overexpressed α-synuclein, possibly through increasing lysosomal GC activity. In surviving DA neurons of human PD midbrain, LIMP-2 levels were increased, probably to compensate for lysosomal GC deficiency. Therefore, we suggest that manipulating LIMP-2 expression to increase lysosomal GC activity is a promising strategy for the treatment of synucleinopathies.
Keywords: AMRF; C57/BL6-J; GD; PME; SCARB2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Reczek D, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–783. - PubMed
-
- Balreira A, et al. A nonsense mutation in the LIMP-2 gene associated with progressive myoclonic epilepsy and nephrotic syndrome. Hum Mol Genet. 2008;17(14):2238–2243. - PubMed
-
- Gamp AC, et al. LIMP-2/LGP85 deficiency causes ureteric pelvic junction obstruction, deafness and peripheral neuropathy in mice. Hum Mol Genet. 2003;12(6):631–646. - PubMed
-
- Dibbens LM, et al. SCARB2 mutations in progressive myoclonus epilepsy (PME) without renal failure. Ann Neurol. 2009;66(4):532–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

