Introduction of sequential inactivated polio vaccine-oral polio vaccine schedule for routine infant immunization in Brazil's National Immunization Program
- PMID: 25316829
- PMCID: PMC4758462
- DOI: 10.1093/infdis/jit588
Introduction of sequential inactivated polio vaccine-oral polio vaccine schedule for routine infant immunization in Brazil's National Immunization Program
Abstract
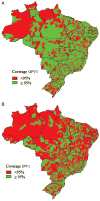
In August 2012, the Brazilian Ministry of Health introduced inactivated polio vaccine (IPV) as part of sequential polio vaccination schedule for all infants beginning their primary vaccination series. The revised childhood immunization schedule included 2 doses of IPV at 2 and 4 months of age followed by 2 doses of oral polio vaccine (OPV) at 6 and 15 months of age. One annual national polio immunization day was maintained to provide OPV to all children aged 6 to 59 months. The decision to introduce IPV was based on preventing rare cases of vaccine-associated paralytic polio, financially sustaining IPV introduction, ensuring equitable access to IPV, and preparing for future OPV cessation following global eradication. Introducing IPV during a national multivaccination campaign led to rapid uptake, despite challenges with local vaccine supply due to high wastage rates. Continuous monitoring is required to achieve high coverage with the sequential polio vaccine schedule.
Keywords: Brazil; acute flaccid paralysis; inactivated polio vaccine; poliomyelitis; vaccination schedule.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Conflict of interest statement
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
Figures
Similar articles
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
-
Poliomyelitis prevention in the United States: introduction of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 1997 Jan 24;46(RR-3):1-25. MMWR Recomm Rep. 1997. PMID: 9026708
-
Introduction of inactivated poliovirus vaccine leading into the polio eradication endgame strategic plan; Hangzhou, China, 2010-2014.Vaccine. 2017 Mar 1;35(9):1281-1286. doi: 10.1016/j.vaccine.2017.01.034. Epub 2017 Feb 1. Vaccine. 2017. PMID: 28161421
-
Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States.Pediatr Clin North Am. 2000 Apr;47(2):287-308. doi: 10.1016/s0031-3955(05)70208-x. Pediatr Clin North Am. 2000. PMID: 10761505 Review.
-
Budget impact of polio immunization strategy for India: introduction of one dose of inactivated poliomyelitis vaccine and reductions in supplemental polio immunization.Public Health. 2017 Jan;142:31-38. doi: 10.1016/j.puhe.2016.10.016. Epub 2016 Nov 17. Public Health. 2017. PMID: 28057194
Cited by
-
Assessing Inactivated Polio Vaccine Introduction and Utilization in Kano State, Nigeria, April-November 2015.J Infect Dis. 2017 Jul 1;216(suppl_1):S137-S145. doi: 10.1093/infdis/jix044. J Infect Dis. 2017. PMID: 28838186 Free PMC article.
-
Administering Multiple Injectable Vaccines During a Single Visit-Summary of Findings From the Accelerated Introduction of Inactivated Polio Vaccine Globally.J Infect Dis. 2017 Jul 1;216(suppl_1):S152-S160. doi: 10.1093/infdis/jix054. J Infect Dis. 2017. PMID: 28838188 Free PMC article. Review.
-
Low Rates of Poliovirus Antibodies in Primary Immunodeficiency Patients on Regular Intravenous Immunoglobulin Treatment.J Clin Immunol. 2018 Jul;38(5):628-634. doi: 10.1007/s10875-018-0531-x. Epub 2018 Jul 14. J Clin Immunol. 2018. PMID: 30006913
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
-
Lessons Learned From Managing the Planning and Implementation of Inactivated Polio Vaccine Introduction in Support of the Polio Endgame.J Infect Dis. 2017 Jul 1;216(suppl_1):S15-S23. doi: 10.1093/infdis/jix185. J Infect Dis. 2017. PMID: 28838203 Free PMC article.
References
-
- World Health Organization. Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Wkly Epidemiol Rec. 2010;85:213–28. - PubMed
-
- Pan American Health Organization. Final report of the XVIII Technical Advisory Group (TAG) meeting on vaccine-preventable diseases of the Pan American Health Organization. Washington, DC: Pan American Health Organization; 2011. Immunization: prioritizing vunerable populations; pp. 15–6.
-
- Ministry of Health. Série C Projetos e Programas e Relatórios. Brasília, Brazil: Ministry of Health; 2003. Programa Nacional de Imunizações—30 anos.
-
- Ministry of Health. Informe Técnico da Introdução da Vacina Inativada Poliomielite. Departamento de Vigilância Epidemiológica; [Accessed 1 October 2013]. http://portal.saude.gov.br/portal/arquivos/pdf/informe_introducao_vacina....
-
- Risi JB., Jr The control of poliomyelitis in Brazil. Rev Infect Dis. 1984;6 (suppl 2):S400–3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

