Deletion of CGI-58 or adipose triglyceride lipase differently affects macrophage function and atherosclerosis
- PMID: 25316883
- PMCID: PMC4242449
- DOI: 10.1194/jlr.M052613
Deletion of CGI-58 or adipose triglyceride lipase differently affects macrophage function and atherosclerosis
Abstract
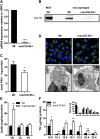
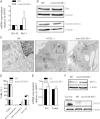
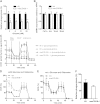
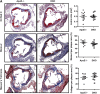
Cellular TG stores are efficiently hydrolyzed by adipose TG lipase (ATGL). Its coactivator comparative gene identification-58 (CGI-58) strongly increases ATGL-mediated TG catabolism in cell culture experiments. To investigate the consequences of CGI-58 deficiency in murine macrophages, we generated mice with a targeted deletion of CGI-58 in myeloid cells (macCGI-58(-/-) mice). CGI-58(-/-) macrophages accumulate intracellular TG-rich lipid droplets and have decreased phagocytic capacity, comparable to ATGL(-/-) macrophages. In contrast to ATGL(-/-) macrophages, however, CGI-58(-/-) macrophages have intact mitochondria and show no indications of mitochondrial apoptosis and endoplasmic reticulum stress, suggesting that TG accumulation per se lacks a significant role in processes leading to mitochondrial dysfunction. Another notable difference is the fact that CGI-58(-/-) macrophages adopt an M1-like phenotype in vitro. Finally, we investigated atherosclerosis susceptibility in macCGI-58/ApoE-double KO (DKO) animals. In response to high-fat/high-cholesterol diet feeding, DKO animals showed comparable plaque formation as observed in ApoE(-/-) mice. In agreement, antisense oligonucleotide-mediated knockdown of CGI-58 in LDL receptor(-/-) mice did not alter atherosclerosis burden in the aortic root. These results suggest that macrophage function and atherosclerosis susceptibility differ fundamentally in these two animal models with disturbed TG catabolism, showing a more severe phenotype by ATGL deficiency.
Keywords: comparative gene identification-58; inflammation; lipid droplets; storage diseases.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- McLaren J. E., Michael D. R., Ashlin T. G., Ramji D. P. 2011. Cytokines, macrophage lipid metabolism and foam cells: implications for cardiovascular disease therapy. Prog. Lipid Res. 50: 331–347. - PubMed
-
- Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab. 3: 309–319. - PubMed
-
- Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R. 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297: E289–E296. - PubMed
-
- Radner F. P., Streith I. E., Schoiswohl G., Schweiger M., Kumari M., Eichmann T. O., Rechberger G., Koefeler H. C., Eder S., Schauer S., et al. 2010. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J. Biol. Chem. 285: 7300–7311. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

