Counterregulation of insulin by leptin as key component of autonomic regulation of body weight
- PMID: 25317239
- PMCID: PMC4138585
- DOI: 10.4239/wjd.v5.i5.606
Counterregulation of insulin by leptin as key component of autonomic regulation of body weight
Abstract
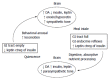
A re-examination of the mechanism controlling eating, locomotion, and metabolism prompts formulation of a new explanatory model containing five features: a coordinating joint role of the (1) autonomic nervous system (ANS); (2) the suprachiasmatic (SCN) master clock in counterbalancing parasympathetic digestive and absorptive functions and feeding with sympathetic locomotor and thermogenic energy expenditure within a circadian framework; (3) interaction of the ANS/SCN command with brain substrates of reward encompassing dopaminergic projections to ventral striatum and limbic and cortical forebrain. These drive the nonhomeostatic feeding and locomotor motivated behaviors in interaction with circulating ghrelin and lateral hypothalamic neurons signaling through melanin concentrating hormone and orexin-hypocretin peptides; (4) counterregulation of insulin by leptin of both gastric and adipose tissue origin through: potentiation by leptin of cholecystokinin-mediated satiation, inhibition of insulin secretion, suppression of insulin lipogenesis by leptin lipolysis, and modulation of peripheral tissue and brain sensitivity to insulin action. Thus weight-loss induced hypoleptimia raises insulin sensitivity and promotes its parasympathetic anabolic actions while obesity-induced hyperleptinemia supresses insulin lipogenic action; and (5) inhibition by leptin of bone mineral accrual suggesting that leptin may contribute to the maintenance of stability of skeletal, lean-body, as well as adipose tissue masses.
Keywords: Autonomic; Circadian; Insulin; Leptin; Weight regulation.
Figures

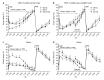
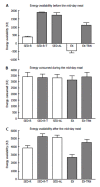
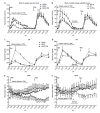
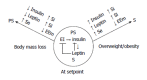
References
-
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief. 2013;(131):1–8. - PubMed
-
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. - PubMed
-
- Sclafani A, Springer D. Dietary obesity in adult rats: Similarity to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–471. - PubMed
-
- Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. - PubMed
-
- Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu Rev Psychol. 2000;51:255–277. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

